Research Article
Protective functions of AEURA in Cell Based Model of Stroke and Alzheimer disease

Jigar Modi1,3 Ahmed Altamimi1, Ashleigh Morrell1, Hongyuan Chou1, Janet Menzie1, Andrew Weiss4, Michael L Marshall4, Andrew Li1, Howard Prentice1-3* and Jang-Yen Wu1-3*
1Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431, USA
2Program in Integrative Biology, Florida Atlantic University, Boca Raton, FL 33431, USA
3Center of Complex Systems and Brain Sciences, Florida Atlantic University, Boca Raton, FL 33431, USA
4AEURA Trust, 2525 Arapahoe Ave E4-138, Boulder, Colorado 80302, USA
*Address for Correspondence: Howard Prentice, PhD, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431, Tel: 561-297-0362; Fax: 561-297-2221; Email: [email protected]
Jang-Yen Wu, PhD, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431, Tel: 561-297-0167; Fax: 561-297-2221; Email: [email protected]
Dates: Submitted: 04 May 2017; Approved: 05 June 2017; Published: 06 June 2017
How to cite this article: Modi J, Altamimi A , Morrell A, Chou H, Menzie J, et al. Protective functions of AEURA in Cell Based Model of Stroke and Alzheimer disease. J Neurosci Neurol Disord. 2017; 1: 016-023. DOI: 10.29328/journal.jnnd.1001003
Copyright License: © 2017 Modi J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Alzheimer’s disease; Stroke; AEURA; Glutamate; Hypoxia; Amyloid Beta (A-Beta)
ABSTRACT
Stroke and neurodegenerative diseases including Alzheimer’s disease (AD) are responsible for a major proportion of mortalities in the elderly. We have previously investigated novel mechanism-based therapies of AEURA in cell culture models against viral infection and in glutamate excitotoxity. In our new studies, we propose that the homeopathic formula AEURA could serve as a potential therapeutic agent for stroke & for AD. In examining AEURA treatment of PC12 cells exposed to glutamate excitotoxicity, hypoxia /re-oxygenation injury and A-Beta toxicity. We demonstrated an increased survival rate in AEURA treated cells by comparison to control cells. In examining the therapeutic potential of AEURA in PC12 cells this homeopathic agent was found to be neuroprotective against either glutamate induced toxicity, hypoxia /re-oxygenation stress or cell stress resulting from viral infection (with either HSV-1 or rhinovirus). Our ongoing studies involve examining the neuroprotective potential AEURA in vivo using rodent models of stroke & AD.
INTRODUCTION
Stroke
A stroke occurs when blood supply is blocked to part of the brain or when a blood vessel in the brain ruptures. In both cases, parts of the brain become damaged or die. A stroke can cause permanent brain damage, long-term disability, or even mortality [1]. Stroke is the 5th leading cause of death in the US, with one person dying every 4 minutes. For African Americans, stroke is the 3rd leading cause of death (Xu et al., 2007). Stroke is also a leading cause of serious long-term incapacity. Stroke decrease mobility in more than half of stroke survivors age 65 and over. Roughly 800,000 people have a stroke each year; almost one every 40 seconds. About 87% of all strokes are ischemic strokes, in which blood flow to the brain is restricted or blocked [2].
An experimental study has revealed that more than 60% of patients develop hypoxia within the first 60 hours after stroke [3]. Stroke or ischemia leads to an increase in the extracellular concentrations of excitatory amino acids, mainly in glutamate [4,5]. This increase in glutamate could be allied to increasing release from neurons, resulting from energy failure, or to reducing clearance of glutamate by glial transporters. An increased production of free radicals and other reactive species in stroke leads to oxidative stress [6]. Stroke (ischemia) causes a cascade of events that can induce glutamate release and increased free radical production via several different pathways [7]. Since these causes, such as increased excitotoxicity, calcium overload, formation of free radicals and inhibition of protein synthesis are all involved in stroke [8], it is a rational strategy to target to treat stroke by calcium antagonists, glutamate antagonists and antioxidants, etc. Despite wonderful efforts in stroke research and major improvements in stroke care within the last decade, therapy is still unsatisfactory. Stroke, exemplified as an undertreated disease, demands an improvement of the existing therapy and a brisk search for new remedies [9].
Alzehimer disease
Alzheimer’s disease is a neurodegenerative illness that is presently affecting more than five million Americans, and it is the 6th leading cause of death in the United States of America [10,11]. 5 million Americans were suffering from Alzheimer’s disease in 2013. At the age of 60, the first symptoms of Alzheimer’s disease appear and the risk increases with age. Younger people may get Alzheimer’s disease, but this is less common. The disease is the 5th leading cause of death among adults aged 65-85 years [10,12]. The number of people with the disease doubles every 5 years beyond age 65. By 2050, this number is projected to rise to 14 million, a nearly three-fold increase [13].
Accumulation of senile plaques is the neuropathological characteristic in brains of Alzheimer’s disease (AD) patients, and the extracellular lesions are primarily comprised of β-amyloid (Aβ). Proteolytic processing converts amyloid precursor protein (APP) into 40 or 42 amino acid peptide which is known as Aβ. Genetic and neuropathological studies provide strong support for a central role of Aβ in the pathogenesis of AD [14]. The pathophysiology of this disease is centered on the extracellular buildup of senile plaques containing amyloid beta. Despite extensive research, there is currently no effective intervention for preventing the progression of the diseases.
AEURA
AEURA is a homeopathic formula currently in use for herpes and comprising multiple components that include: Mezereum (plant derived), Phytolacca decendra (plant derived), Ranunculus bulbosus (plant derived) and Rhus toxicodendron (plant derived) [15]. The AEURA formula is natural anti-viral over the counter (OTC) sublingual homeopathic medicine proposed to boost the immune system and treat the symptoms of Herpes, Cold Sores and Shingles [15,16]. AEURA does not require doctors’ visits or prescriptions and is safe for children [15]. AEURA is Homeopathic formula and has been found to display antiviral properties in culture and in herpes simplex virus (HSV) infected patients treated by this medication [15,16]. A frequently used neuronal cell culture model is the pheochromocytoma PC-12 cell line, a cell line derived from adrenal chromaffin cells, in which we have analyzed the analyzed the ability of AEURA to decrease toxicity associated with herpes simplex virus infection. Acyclovir is known drug for HSV 1 and it added to killing of HSV-1 infected cells but AEURA was protective against HSV-1 induced cell death [15]. In our previous study, we investigated the effect of AEURA on PC12 cells with glutamate 5mM treatment. In the absence of AEURA, we found in approximately 50% cell death in normal cells. AEURA was highly protective against glutamate toxicity in the range of 40 ng/ml to 4 micro-grams/ml with maximal protection at 640 ng/ml AEURA [16].
Each 300 mg tablet of AEURA contains as an inactive ingredient: Magnesium stearate USP 1% [15]. In our ongoing studies of neuroprotection by AEURA, we are fractionating the compound based on charge and molecular weight. We used liquid AEURA with original active ingredients for experiment compare to our previous work on tablet form of AEURA [16]. We propose in our future work to evaluate active fractions of AEURA for cellular protection against glutamate excitotoxicity, hypoxia re-oxygenation stress and A-Beta toxicity.
Many neurological disorders and neurodegenerative diseases including Alzheimer’s, Stroke, Parkinson’s and Huntington’s diseases disclose a common essential pathophysiology of glutamate excitotoxicity, calcium imbalance or oxidative stress which individually or jointly results in cell death (Mohammad-Gharibani et al., 2014). Therefore, AEURA’s role as an inducer of inhibitory neurotransmission, an anti-oxidant, neuromodulator, regulator of calcium homeostasis and neuroprotectant makes it an ideal therapeutic agent for many of these diseases. This study will focus on previous and current studies of AEURA’s major effects, with a primary focus on stroke and Alzheimer’s disease.
MATERIALS AND METHODS
Materials
F-12K media, trypsin-EDTA solution, horse serum and rat pheochromocytoma (PC). PC12 cell line were purchased from ATCC (Manassas, VA, USA). Fetal bovine serum, poly-D-lysine and other chemicals like Glutamate, Amyloid Beta protein fragment (A-Beta) were purchased from Sigma (St. Louis, MO, USA). Adenosine 5’-triphosphate (ATP) Bioluminescent Assay Kit was purchased from Promega (Madison, WI, USA).
Cell culture
PC12 cell line culture: PC-12 cells were cultured in petri dish for one week prior to cell use. PC12 cells were maintained at 37oC /5% CO2 in incubator and Cells were fed every other day using F12-K medium supplemented with 5% (v/v) fetal bovine serum (FBS), 10% (v/v) heat-inactivated horse serum (HS) and 1% (v/v) penicillin-streptomycin solution. All experiments were performed on undifferentiated cells plated in 96-well plates at a density of approximately 5×10⁴ cells/ml for the ATP assay [17]. On first day of use, cells were harvested by first adding 2 mL Trypsin and incubated for 15 minutes. 2mL fresh medium was then used as a wash and cells were spun, re-suspended and counted Counts were estimated as 25,000 cells per well in 96 well plate where cells were transferred, then incubated for 24 hour and varying AEURA concentrations were added along with 10 mM Glutamate/ 100 uM Cocl2/ 20uL of A-Beta.
Glutamate toxicity
For glutamate-induced toxicity, Cell culture in vitro were preincubated with a proper appropriate concentration of AEURA for one hour. Then the neurons were treated with glutamate (10 mM) for 24 hour [16].
Hypoxia/reoxygenation
To generate hypoxic conditions, PC-12 cells in 6 or 96 well plates were placed in a hypoxia chamber with oxygen levels maintained at 0.3-0.4 % [8,17]. The level of oxygen was continuously monitored using an oxygen electrode. PC-12 cell cultures in the absence or presence of AEURA were subjected to 24 hours of hypoxia. Reoxygenation was performed by removing cultured plates from the hypoxic chamber and transferring them into normal culture incubator remaining for another 24 hours.
A-Beta toxicity
For A-Beta induced toxicity, Cell culture in vitro were preincubated with a proper concentration of AEURA for one hour. Then the neurons were treated with assigned concentration of A-Beta (20 uM) for proper time 24 hour.
AEURA concentration
Concentrations of AEURA listed for experimental testing of liquid AEURA were based on the minimum equivalent dilutions of tablet AEURA employed to obtain the identical degree of protection.
Measurement of cell viability by ATP assay
PC-12 cells in 96-well plates were treated with or without AEURA for 1 hour, and then cells were subjected to Glutamate / hypoxia–re-oxygenation conditions / CoCl2 / A-Beta for 24 hour to induce cell death. ATP solution was added to each well, and cells were incubated for 10 min after which the amount of ATP was quantified through a luciferase reaction. The luminescence intensity was determined using a luminometer with lysates in a standard opaque-walled multi-well plate. The ATP content was determined by running an internal standard and expressed as a percentage of untreated cells (control) [8,17].
Statistical analysis
All data were expressed as the mean ± SEM. A computer program (SPSS 15.0, Chicago, IL, USA and Prism Graph Pad 7) was used for statistical analysis. The statistical significance of the data was determined with t-test or one-way ANOVA combined with Dunnett post-hoc or Tukey test for comparison between groups. Differences of P<0.05 were considered statistically significant. At least three independent replicates were performed for each experiment.
RESULTS
Glutamate excitotoxicity is dose-dependent in PC-12 cell culture
The PC12 cells were exposed to different concentrations of glutamate in a range of 0.01 mM to 150 mM for 24 hour, then the ATP assay was performed (Data not shown). As expected, the survival of PC12 cells decreased with the increasing of concentrations of glutamate. We chose the optimal doses of 10 mM glutamate for cell viability test [18]. At 10 mM glutamate, about 55% of survival PC12 cells was observed from 76% at 10 μM glutamate to 18% at 150 mM glutamate.
Effect of AEURA on glutamate excitotoxicity
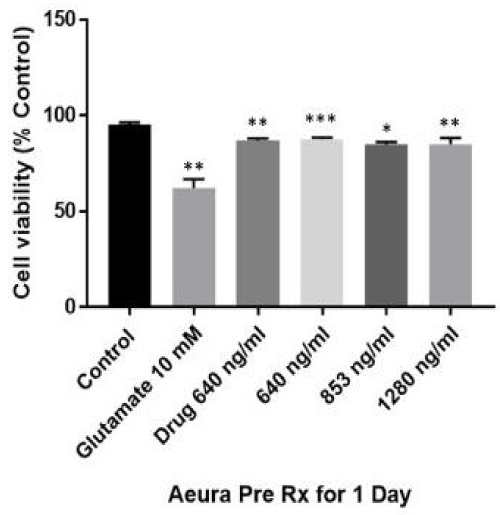
Levels of protection against glutamate toxicity were directly compared using dissolved tablet by comparison to dilutions of stock liquid AEURA. The AEURA concentrations employed were covered the range from minimum required for protection of PC12 cells against glutamate toxicity which was 640 ng/ml (1 in 3600 dilution) to 853 ng/ml (1 in 2700 dilution) to 1280 ng/ml (1 in 1800 dilution). In our experiment, the viability of cells exposed to10 mM glutamate for 24 hour was 59.6 % of the control value, while the viability of cells that were pretreated for 24 hour with different concentrations of AEURA (640, 853 & 1280 ng/ml) prior to exposure to glutamate significantly increased from 87.71% and 84.93% to 83.2% of the control value, respectively (Figure 1).
Figure 1: AEURA enhances PC 12 cell survival under glutamate (10 mM) exposure. PC-12 cells were pretreated with AEURA for 24 hour. Then AEURA was removed and replaced by cell medium and keep it for 24 hour and then exposed to 10mM glutamate for 24 hour. Cell viability measured using ATP assay. Control values were fixed at 100%. The values for glutamate and AEURA + glutamate were normalized relative to the control values and represent mean ± SEM of 5 preparations. (All data are presented as mean +/- SEM, where *P < 0.05, **P < 0.01, ***P < 0.001).
Neuroprotective effects of AEURA on PC-12 cell culture under hypoxia/reoxygenation condition
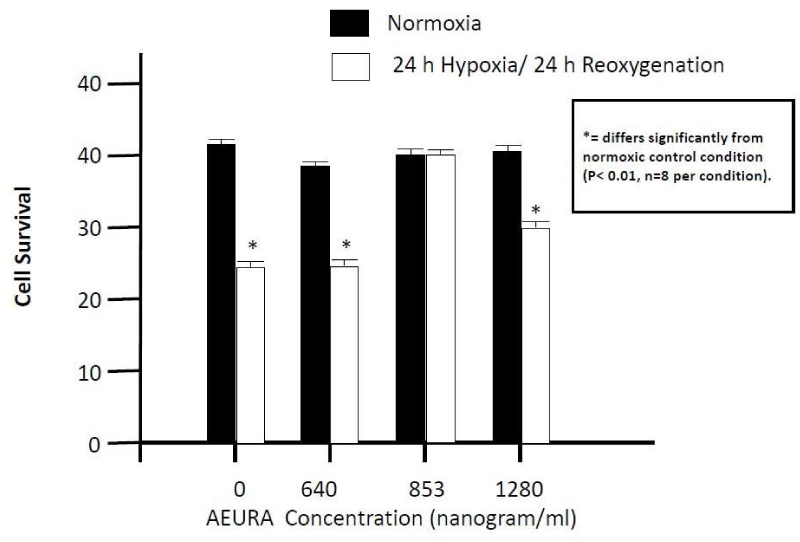
To determine the appropriate concentration of AEURA in cultures, PC-12 cells were exposed to hypoxia and reoxygenation in the presence or in the absence of 640 - 1280 ng/ml AEURA as shown in figure 2. In hypoxia/re-oxygenation doses of 640 ng/ml (1 in 3600 dilution), 853 ng/ml (1 in 2700 dilution) and 1280 ng/ml (1 in 1800 dilution) were employed. After hypoxia and reoxygenation, ATP levels for PC-12 cells without AEURA treatment dropped to about 59% (percentage of control). AEURA treatment dramatically increases the cell viability. The presence of 853 ng/ml AEURA clearly improved the cell viability to greater than 90%. With increasing AEURA concentrations up to 1280 ng/ml cell viability increased to 75%.
Figure 2: Effect of AEURA on PC12 cell survival following 24-hour Hypoxia followed by 24 hour reoxygenation. PC-12 cells were pretreated with AEURA for 1 hour and then expose to 24-hour hypoxia followed by 24 hour reoxygenation. Cell viability measured using ATP assay. Control values were fixed at 100%. The values for Hypoxia and AEURA + Hypoxia were normalized relative to the control values and represent mean ± SEM of 5 preparations. (All data are presented as mean+/-SEM, where *P < 0.01).
Effect of A-Beta on PC-12 cell culture
To evaluate the A-beta toxicity in PC-12 cell culture, neurons were exposed to different concentration of A-Beta ranging from 1 μM to 40 μM for 24 hour, then the ATP assay was performed (data not shown).
For analysis of AEURA effects on A-beta toxicity concentrations of liquid AEURA employed started at 640 ng/ml (1 in 3600 dilution) which was chosen on the base of being the minimum concentration of AEURA in tablet form needed for protection against glutamate toxicity. Doses employed were 640 ng/ml (1 in 3600 dilution of stock), 1280 ng/ml (1 in 1800 dilution), 1706 ng/ml (1 in 1350 dilution), 2560 ng/ml (1 in 900 dilution) and 5120 ng/ml (1 in 450 dilution of stock).
Cell viability decreases with the elevation of A-Beta concentration and ATP levels at 40 μM glutamate significantly dropped to approximately 75%, compared to control neurons. With A-Beta concentration of 10 μM cell viability decreased to about 24% of the control neurons. Cell survival at 5 μM A-Beta is very close to that at 1μM, the cell viability at 20 μM A-Beta reduces to 46% of the controls. Thus, 20 μM A-Beta was employed for neuronal excitotoxicity at different time points.
AEURA demonstrates strong neuroprotective action against A-Beta (Aβ) toxicity on PC-12 cell cultures
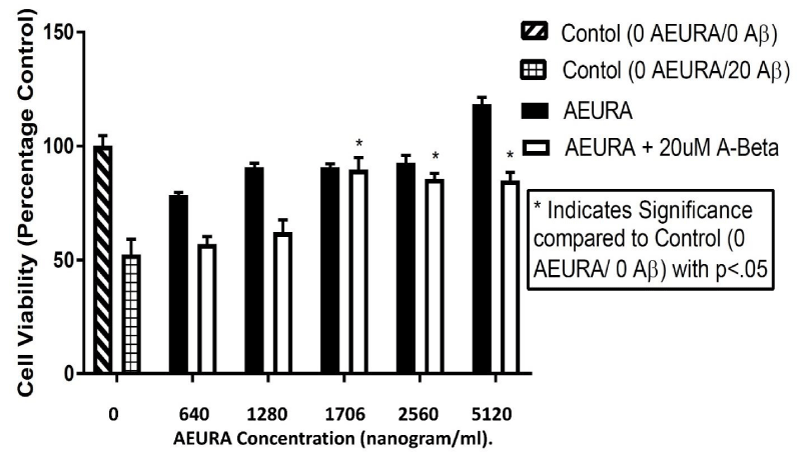
In our study, we tested the effect of AEURA on the PC-12 cell culture in A-Beta (Aβ -20uM dose) condition. Our data showed that 640 - 5120 ng/ml AEURA can attenuate cell death in A-Beta toxicity. After A-Beta treatment, viability of cells without AEURA treatment dropped to approximately 51 % of control. AEURA treatment of 1706, 2560 & 5120 ng/ml dramatically increased the cell viability to almost 89.10, 82.37 & 80 % of the control level (Figure 3).
Figure 3: AEURA enhances viability of PC12 cells effected by Aβ toxicity. Cells were exposed to Aβ (20uM) and treated with AEURA. After 24-hours the cells were lysed and an ATP assay was performed to determine cell viability. Control values were fixed at 100%. The values for Aβ and AEURA + Aβ were normalized relative to the control values and represent mean ± SEM of 5 preparations. (All data are presented as mean+/-SEM, where *P < 0.05, **P < 0.01, ***P < 0.001).
DISCUSSION
The present analysis demonstrates that AEURA, a neuroprotective agent with anti-viral properties [16], exerted significantly neuroprotective effects against either glutamate-induced cytotoxicity, or hypoxic injury or A-Beta toxicity in PC12 cells in a dose-dependent manner. Even in the long-term glutamate-induced cytotoxicity or hypoxic injury or A-Beta toxicity in PC12 cells, a significantly neuroprotective effect of AEURA was found. Also, the cellular and molecular mechanisms that trigger the action of AEURA have not yet been fully explained.
The key mechanism of neuronal death in cerebral ischemia/ Stroke is glutamate toxicity [19]. Two mechanisms of glutamate neurotoxicity are studied in neuron injury, one is glutamate receptor-mediated [22] and the other is oxidative stress-mediated [20,21] neurotoxicity. We previously demonstrated that AEURA protect normal PC-12 cells as well as cells under conditions of glutamate excitotoxicity 5mM for 4 hours [16].
In this paper, ATP levels are drastically improved by AEURA relative to hypoxia/reoxygenation or glutamate treatment or A-beta treatment alone, indicating high cell viability and protective effects upon treatment with AEURA in these three toxic conditions.
In our study, pretreatment with AEURA shows a protective effect against hypoxia and a glutamate-induced cytotoxicity in PC 12 cells probably through oxidative stress amelioration.
The primary mechanism of AEURA action is to protect against glutamate induced toxicity, a pro-survival process which involves preventing calcium overload either through inhibiting calcium influx from extracellular sources or from inhibiting release of calcium from intracellular stores. Down-stream of calcium overload, essential effects for enhancing cell survival in the face of either hypoxia/re-oxygenation or A-beta toxicity include preventing induction of pro-apoptotic pathways in addition to ER stress pathways.
In addition to preventing glutamate induced excitotoxicity that would lead to cell death AEURA is likely to stimulate antioxidant activity. Such a potential antioxidant role for AEURA is based on the fact of pre-exposure to the drug may increase cellular tolerance to stress through preconditioning responses, some of which are known to induce antioxidant enzymes such as manganese superoxide dismutase.
An early event in Alzheimer’s disease is the aggregation of beta-amyloid (Aβ) as a soluble oligomer. Importantly, the presence of these aggregates seems to indicate to neurodegeneration in the progression of this disease. However, the mechanisms underlying Aβ-induced neurotoxicity are not absolutely known in primary cultures of pyramidal neurons [23].
PC12 cells represent a commonly established neuronal model system. This cell model is also generally employed to study cellular glutamate toxicity [18]. PC12 cells are highly sensitive to glutamate or hypoxia or A-Beta induced injury, therefore, we assumed that it is appropriate for PC12 to examine whether AEURA offers protection against glutamate or hypoxia or A-Beta induced cytotoxicity. PC12 cells may not fully replicate the phenotype of primary cultured neurons, and for this reason our future work may in addition focus on primary neurons and in vivo models.
The protection afforded by AEURA against A-beta toxicity is likely to induce blocking of both glutamate induced toxicity and calcium overload following A-beat exposure. In addition, downstream effects of A-beta on apoptosis and on ER stress are most likely to be inhibited by AEURA treatment.
CONCLUSION
AEURA, a widely used homeopathic formula [15,16], was shown to have protective function against cell injury/cell death induced by viral infection [16], excitotoxicity, hypoxic condition (a condition mimicking stroke) and A-beta toxicity [a condition mimicking Alzheimer’s disease(AD)]. It is important to note that in these initial studies protection is found in the PC12 cell culture line but for future application to AD and stroke additional tests will be necessary to demonstrate potential clinical applicability.
ACKNOWLEDGEMENTS
The current work was funded in part by grants from the Department of Health, State of Florida and by a grant from the AEURA Trust.
REFERENCES
- Kwiatkowski T, Libman R, Tilley BC, Lewandowski C, Grotta JC, et al. The impact of imbalances in baseline stroke severity on outcome in the national institute of neurological disorders and stroke recombinant tissue plasminogen activator stroke study. Ann Emerg Med. 2005; 45: 377-384. Ref.: https://goo.gl/kKHTR2
- Moreau F, Yang R, Nambiar V, Demchuk AM, Dunn JF. Near-infrared measurements of brain oxygenation in stroke. Neurophotonics. 2016; 3. Ref.: https://goo.gl/0J0H1h
- Roffe C. Hypoxaemia and stroke. Rev Clin Gerontol. 2001; 11: 323-335. Ref.: https://goo.gl/O3j4P1
- Dávalos A, Shuaib A, Wahlgren NG. Neurotransmitters and pathophysiology of stroke: evidence for the release of glutamate and other transmitters/mediators in animals and humans. J Stroke Cerebrovasc Dis. 2000; 9: 2-8. Ref.: https://goo.gl/RHWvve
- Prentice H, Modi JP, Wu JY. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev. 2015; 2015: 964518. Ref.: https://goo.gl/Io2Z5N
- Alexandrova M, Bochev P, Markova V, Bechev B, Popova M. Dynamics of free radical processes in acute ischemic stroke: influence on neurological status and outcome. J Clin Neurosci. 2004; 11: 501-506. Ref.: https://goo.gl/oFEakI
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999; 79: 1431-1568. Ref.: https://goo.gl/CqKTVJ
- Mohammad-Gharibani P, Modi J, Menzie J, Genova R, Ma Z. Mode of action of S-Methyl-N, N-Diethylthiocarbamate Sulfoxide (DETC-MeSO) as a novel therapy for stroke in a rat model. Mol Neurobiol. 2014; 50: 655-672. Ref.: https://goo.gl/DZXDLQ
- Lyden P, Wahlgren NG. Mechanisms of action of neuroprotectants in stroke. J Stroke Cerebrovasc Dis. 2000; 9: 9-14. Ref.: https://goo.gl/fPWBGQ
- Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep. 2013; 62: 1-96. Ref.: https://goo.gl/6NfRh2
- Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. Natl Vital Stat Rep. 2010; 58: 1-19. Ref.: https://goo.gl/0yKvUC
- Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep. 2013; 20: 62: 1-96. Ref.: https://goo.gl/FKK7q6
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013; 80: 1778-1783. Ref.: https://goo.gl/ua9lDV
- Chafekar SM, Baas F, Scheper W. Oligomer-specific Aβ toxicity in cell models is mediated by selective uptake. Biochim Biophys Acta-Mol Basis Dis. 2008; 1782: 523-531. Ref.: https://goo.gl/pQg5of
- Products-AEURA [WWW Document], 2008. URL http://aeura.co/products/ (accessed 4.21.17).
- Prentice H, Modi J, Menzie J, Chou H, Weiss A, et al. SciTz neurology and neurosciences neuroprotective mechanisms of action of DETC-Meso, GCSF, sulindac, taurine and AEURA 2016; 1.
- Pan C, Prentice H, Price AL, Wu JY. Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids. 2012; 43: 845-855. Ref.: https://goo.gl/hr3im1
- Ma S, Liu H, Jiao H, Wang L, Chen L. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca2+ influx. Neurotoxicology. 2012; 33: 59-69. Ref.: https://goo.gl/gfLfWL
- Kim JY, Jeong HY, Lee HK, Kim S, Hwang BY. Neuroprotection of the leaf and stem of Vitis amurensis and their active compounds against ischemic brain damage in rats and excitotoxicity in cultured neurons. Phytomedicine. 2012; 19: 150-159. Ref.: https://goo.gl/sAbGZv
- Chen J, Chua KW, Chua CC, Yu H, Pei A. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci Lett. 2011; 499: 181-185. Ref.: https://goo.gl/a4M8rg
- Hirata Y, Yamamoto H, Atta MS, Mahmoud S, Oh-hashi K. Chloroquine inhibits glutamate-induced death of a neuronal cell line by reducing reactive oxygen species through sigma-1 receptor. J Neurochem. 2011; 119: 839-847. Ref.: https://goo.gl/NyE2pY
- Kanki R, Nakamizo T, Yamashita H, Kihara T, Sawada H. Effects of mitochondrial dysfunction on glutamate receptor-mediated neurotoxicity in cultured rat spinal motor neurons. Brain Res. 2004; 1015; 73-81. Ref.: https://goo.gl/SozTMn
- Ferreira A, Sinjoanu RC, Nicholson A, Kleinschmidt S. Aβ toxicity in primary cultured neurons. 2010; 141-153. Ref.: https://goo.gl/zXyDCB