More Information
Submitted: 22 July 2019 | Approved: 30 July 2019 | Published: 31 July 2019
How to cite this article: Luisetto M, Ahmadabadi BN, Rafa AY, Sahu RK, Cabianca L, et al. The turing machine theory for some spinal cord and brain condition, A toxicological - antidotic depurative approach. J Neurosci Neurol Disord. 2019; 3: 102-134.
DOI: 10.29328/journal.jnnd.1001023
Copyright License: © 2019 Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Spinal cord; Brain; Degenerative disease; Trauma; Immune disease; Neurotoxicity pathology; Toxicology; Pharmacology; Depurative methods; Informatics; Algorytms
The turing machine theory for some spinal cord and brain condition, A toxicological - antidotic depurative approach
Mauro Luisetto1*, Behzad Nili Ahmadabadi2, Ahmed Yesvi Rafa3, Ram Kumar Sahu4, Luca Cabianca5, Ghulam Rasool Mashori6 and Farhan Ahmad Khan7
1Applied Pharmacologist, European Specialist Lab, Branch General Toxicology, Italy
2Innovative Pharmaceutical Product Development Specialist, USA
3Founder and President Yugen Research Organization, Bangladesh
4Ram Kumar Sahu, Pt Deendayal Upadhyay Memorial Health Science and Ayush University of Chhattisgarh, India
5Medical Laboratory, Italy
6Department of Medical & Health Sciences for Woman, Peoples University of Medical and Health Sciences for Women, Pakistan
7Government Medical College and Hospital Shahdol, India
*Address for Correspondence: Mauro Luisetto, Applied Pharmacologist, European Specialist Lab, Medicine, Branch General Toxicology, Italy; Email: [email protected]
Aim of this work is to produce a general theory related an new depurative strategy to be devalued for reduce or delay some spinal cord and brain degenerative and inflammatory chronic disease or acute traumatic condition. It is used and informatics approach in order to set correct the problem and the process. Scope of this project is to submit to the researcher a new therapeutic strategy (under a depurative- toxicological-pharmacological) in this complex kind of disease. A Turing machine theory say us a method to TRASLATE the need of a strategy in a practical hypotesys of work. A global conceptual map can help in this field.
Before to start this work is crucial to verify what happen in other field like botany
In article Amyotropyc Lateral Sclerosis and Endogenous -Exogenous Toxicological Movens: New model to verify other Pharmacological Strategies 2018 is reported that: “This work start from some question related neuro-degenerative disease and related other science: in article Brain and immune system: KURU disease a toxicological process was written observing also the different degenerative brain disease, with accumulation we can verify if exist an immune systems role. (AD, Parkinson disease, Lewy Body Dementia, Pick disease and other CNS amyloidosis) Neurodegenereative Protein Related Disease (tau-patie), with brain accumulation and interference with many cognitive functions. Are there similarity in some neuro-degenerative pathology like Tua-patie, alfa – sincucleino-paty and CJD, prions disease? And related to other progressive dementias such as Alzheimer’s and PD, ALS? (Catabolic- cumulated immune toxic mediated process?) Is universally know that in example some plants produces alckaloids as bioproducts in their metabolism not having excretor apparatus as other animal organism. Can we consider waste of immune systems some accumulation substanties’ in some brain Pathology? (Materials that cannot leave from central nervous system: A global catabolic- afinalistic process?) Observing this scientific - literature we can say that some neurologic disease can present common aspect: Accumulation of some metabolic- catabolic toxic substantia and related to the progression of disease and involved with immune system activation.” Some question can be useful to the scope of this research. Why in example different region of brain (and different function) are involved in the different neuro-degenerative- inflammatory pathology? In example DA-cortical-cognitive, Parkinson Disease in basal nuclei–movement, ALS often spinal cord involvement–progressive paralisys? Why in dementia are more involved cortical neuron (and hippocampus) vs spinal? There is a gradient (top-bottom)? The selective sensibility of the various type of neurons or other factors can influence the process? Other question: in many neuro-degenerative disease there is an increase of some catabolic-metabolic products that can produce or are related to the neuro-degeneration (DA, PD et other). And is involved also a kind of “waste system“ of SNC? BEE is a natural barrier that protect SNC from toxic subtantia, but this barrier is also efficacy in reverse sense: from inside SCN to outside? And the role played by cephalo- rachidan liquid? Like a blood and lymphatic role played in other organ. A brain omeostatic fluid. (In brais is absent a lymphatic structure organized). BEE contribute to physiology of the cephalo rachidion fluid composition. Why the space between brain cells may increase during sleep, allowing the brain to flush out toxins that build up during waking hours? It depend by position? Or due by different brain activation level? And what imply human evolution as bipedal position? In brain wasting system efficacy? Related evolution of vertebrates: cortex is a more recent structure than spinal cord, mesencefalus and rombencephaus so is possible to say that the washing central nervous system present a different efficacy in kinetics of the “washing activity” form catabolites?
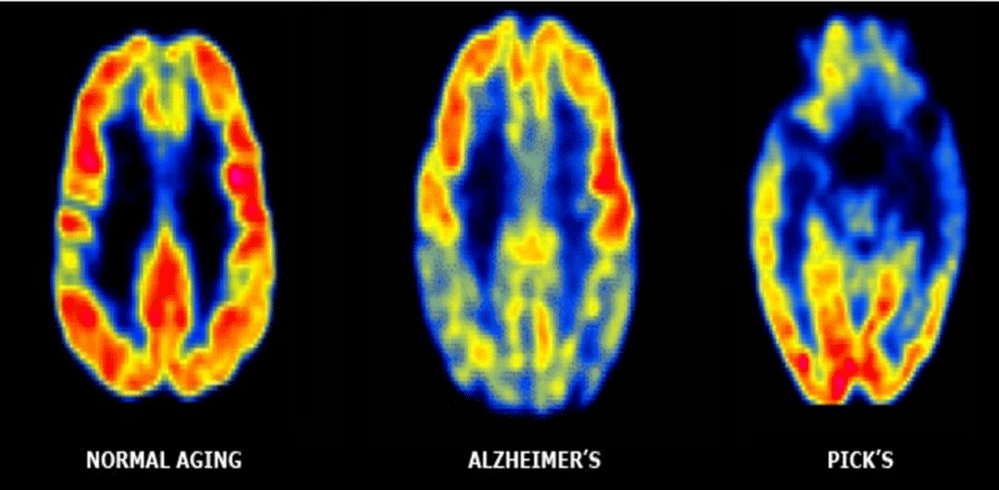
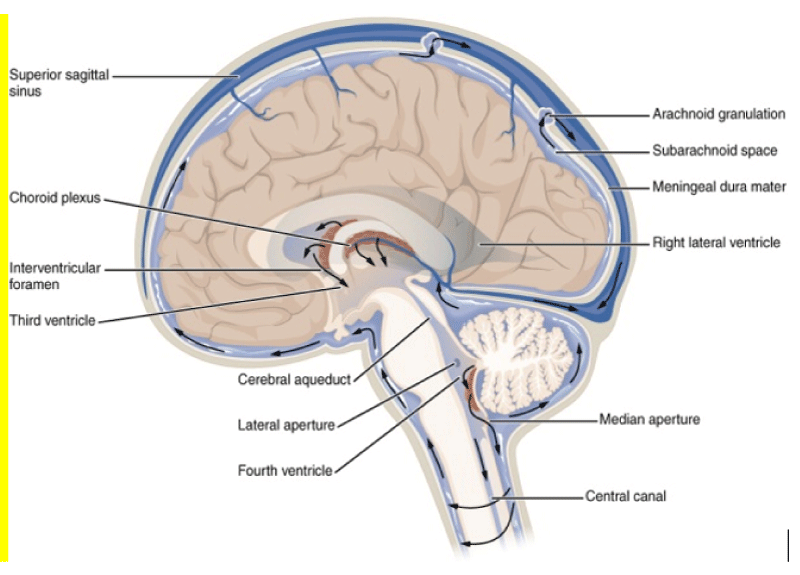
There is a reason why in DA are involved more cortex and hippocampus than spinal cord? In a sort of gradient different from top to bottom? And this can be related to physiology of wasting brain system? In DA cognitive problems are more than other spinal damages effect. BBB present the same barrier properties also from inside of SNC and outside like form external to internal? Normal brain function requires a large amount energy, to supply enough oxygen and remove adequate amounts of wastes for respiration, large networks of capillaries must be constructed in the brain for molecular exchange. A high prevalence of capillaries allows for quick diffusion of reactants and products of respiration, and increases the possibility that other contents in the blood may diffuse across the capillary wall. The BBB evolved to allow for a higher rate of selectivity for what may pass through to the brain tissue to help preserve and protect the brain from possible detrimental molecules or organisms in the blood [1,2]. Scope of this article is to verify some relevant condition involving the “SNC washing system” that can be useful in some neurodegenerative or inflammatory (but also acute trauma) pathology. Some image can efficacy provide example of this argument useful to the discussion: Cerebro-spinal fluid CSF is a fluid and is in SNC whit different function like to reduce the brain weight, to make possible brain perfusion at kostant pressure, but also a Lhympatic like function. It is under proper dynamic movement related heart activity: during systolic from lateral ventricules to 3-4 ventricules, this intrarachidea space and in medullar channel (Figures 1,2). During diastolic phases: opposite direction In Magendie forame and Luschka direction is always from first compartment (intracranic) to the one extracranic (2nd meningeal compartement; the same for 3rd meningeal compartement. Every organ present its wasting system to collect products of catabolism or other substantia so is interesting to observe brain and spinal cord “wasting system“. Bee is a natural barrier the protect SNC from toxic subtstantia but this barrier is also a barrier to the flux in reverse WAY? (From CNS to the out brain environment?) BEFORE produce and research hyotesys is fundamental observe some published article: Korean J Radiol. 2016 Nov-Dec; 17(6): 827–845. According article: Structural MR Imaging in the Diagnosis of Alzheimer’s Disease and Other Neurodegenerative Dementia: Current Imaging Approach and Future Perspectives Mina Par, et al.
Figure 1: Imaging normal vs AD and Picks disease.
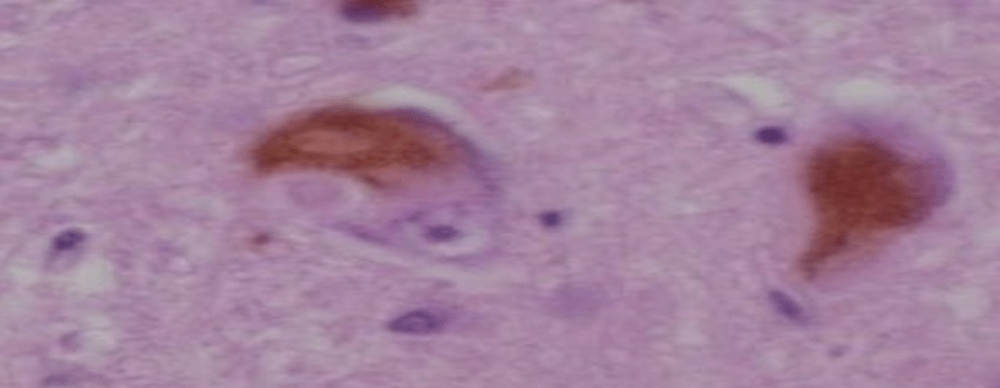
Figure 2: Dementia LEWY BODYES, histology.
Characteristic pathology and pathogenesis model of AD
Alzheimer’s disease is characterized by the accumulation of two abnormal proteins: extracellular Aβ protein and intracellular tau protein [3,4]. Amyloid and tau deposition progress spatiotemporally in a predictive manner. Amyloid first accumulates in the basal part of the frontal, temporal, and occipital lobes, and subsequently spreads to the entorhinal cortex, hippocampus, amygdala, insular cortex, and cingulate cortex, sparing the primary visual and sensorimotor cortices. Conversely, neurofibrillary tangle deposition progresses in the following order: transentorhinal cortex, entorhinal cortex, hippocampus, temporal cortex, association cortices, and finally the primary sensorimotor and visual cortices. The Aβ hypothesis, the dominant theory of AD, suggests that overproduction or inadequate clearance of Aβ is a causative factor for AD; AD begins with the abnormal metabolism of the transmembrane amyloid precursor protein (APP). β and γ-secretases cleave APPs to form several Aβ peptide fragments . Of these, the most important is Aβ 42, which is highly prone to aggregation and resultant plaque formation. Although amyloid deposits are typically observed in the extracellular space, Aβ is also found within neurons, and this may be related to the aggregation of other cellular proteins such as tau protein in AD. Subsequently, abnormal phosphorylation of the microtubule-associated tau protein in neurons and the formation of neurofibrillary tangles are thought to result in the disruption of normal neuronal function. Oxidative and inflammatory stresses from Aβ also contribute to the loss of synaptic and neuronal integrity, and finally, neuronal loss and brain atrophy. This downstream pathological cascade has been re-interpreted by the hypothetical model of Jack et al. Conversely, Braak and Del Tredici observed hyperphosphorylated tau protein in the absence of Aβ deposition in the medial temporal limbic isocortex of young individuals. Furthermore, recent evidence suggests that tau deposition is a requisite for amyloid toxicity in vivo. These findings raise questions regarding the role of Aβ as an initiator of the AD pathophysiological cascade.
Multiple Sclerosis Patients’ Cerebro-spinal Fluid Offers New Clues for Potential Therapeutic Strategies
Studies show that treating live neurons using cerbrospinal fluid from progressive MS patients causes mitochondrial dysfunction. In article: https://www.genengnews.com/news/multiple-sclerosis-patients-cerebro-spinal-fluid-offers-new-insights-into-potential-therapeutic-strategies (Genetic Engineering & Biotechnology News) is reported that: “Multiple Sclerosis Patients’ Cerebro-spinal Fluid Offers New Clues for Potential Therapeutic Strategies: MS is a neuro-degenerative disorder that may take 2 basic forms, relapsing remitting MS (RMMS), which presents with periods of clinical remission, and progressive MS, which is characterized by continued deterioration without remission. There are some therapies available to help manage RRMS, but treating progressive MS is far more challenging. By studying the effects of cerebro-spinal fluid (CSF) from MS patients on mitochondria in mouse neurons, US researchers have now identified a biological mechanism that might ultimately help develop new therapeutic strategies against the progressive form of the disease. “Because the brain is bathed by the CSF, we asked whether treating cultured neurons with the CSF from MS patients with a relapsing/remitting or a progressive disease course would possibly elicit different effects on neuronal mitochondrial function” said P Casaccia, PhD professor of biology at the Graduate Center and founding director of the Neuroscience Initiative at the Advanced Science Research Center (ASRC) at the City University of New York, and the Icahn School of Medicine at M Sinai. “We detected dramatic differences in the shape of the neuronal mitochondria and their ability to produce energy.”Casaccia and coll (Figure 3). reported their findings in Brain, in a paper titled, “A metabolic perspective on CSF-medicated neuro-degeneration in MS. MS is characterized by destruction of the myelin sheath that surrounds nerve cells. RRMS is the most common form of MS and affects about 85% of patients, who exhibit demyelinating inflammatory episodes with clinical symptoms, followed by periods of clinical remission. The 15% of patients who present with primary progressive MS exhibit progressive neurological deterioration without periods of clinical remission. Approximately 50% of RRMS patients will also eventually develop progressive disease. While there are approved immune-modulatory drugs that can help decrease inflammation that is characteristic of RRMS, “progressive MS, with disability driven by neuro-degeneration and inflammation that is intrinsic to the CNS, has been more difficult to manage,” the authors noted.
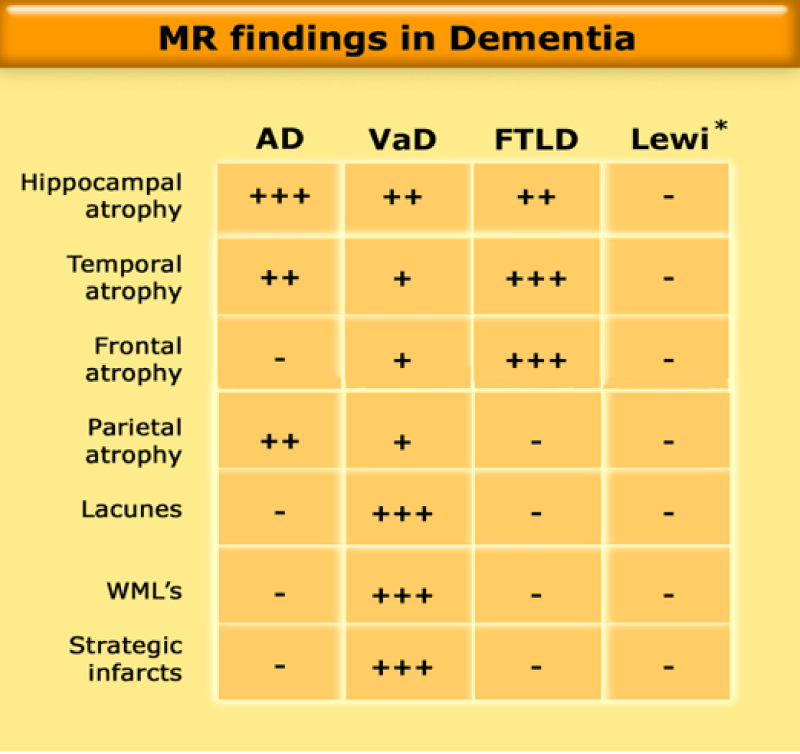
Figure 3: MR findings in Dementia.
One of the greatest challenges for the field of MS remains the therapeutic management of the neuro-degenerative component of the disease. This is likely due to the elusive nature of the molecular mechanisms underlying disease progression, which has precluded the potential definition of effective therapeutic target. ”Previous studies in animals have suggested that dysfunction of mitochondria in nerve cells may be a feature of progressive MS, but the molecular mechanisms underlying the process aren’t known. To look at this in more detail Casaccia and colleagues investigated whether there were any differential effects of treating rat neurons with CSF taken from human RRMS patients or those with progressive MS. The researchers functionally and metabolically characterized CSF samples from 15 patients with RRMS and another 29 with progressive MS, and exposed live cultured rat neurons to the samples. Any effects on the neurons were recorded directly by time-lapse videos using live confocal imaging. A mitochondrial tracer was used to allow visualization of any changes to mitochondria. The videos revealed important differences between the effects of the 2 different CSF sample types. Mitochondria exposed to CSF from progressive MS patients became much more elongated and fused together. “Notably, we detected a substantial elongation of these organelles, coalescing to form a tubular network only in neuronal cultures exposed to the CSF from progressive patients,” the team reported. This response was not seen in mitochondria exposed to CSF from patients with a relapsing/remitting MS. Further biochemical tests showed that the elongated mitochondria didn’t function as well and so were less capable of producing energy, which eventually resulted in neuronal cell death. “We detected dramatic differences in the shape of the neuronal mitochondria and their ability to produce energy,” Casaccia stated. “Only exposure to the CSF from progressive MS patients caused neuronal mitochondria to fuse and elongate while rendering them unable to produce energy. Previous research has suggested that mitochondria elongate in an attempt to generate more energy for cells when there is enhanced energetic demand or a decrease in available glucose. To try and find what might be present in the CSF of progressive MS that triggers this elongation response, the team first destroyed any proteins in the samples by subjecting them to heat, and then retested the heat-treated samples on rat neurons. Interestingly, there was a “remarkable effect” of the CSF from progressive patients on mitochondrial elongation, which the researchers say “ruled out a potential contribution of protein components. They then carried out an analysis of lipid components in the CSF from RRMS patients and from progressive MS patients, and found increased levels of ceramides and particularly ceramide C24, in the progressive MS CSF. Ceramides are sphingolipids that have previously been implicated in MS, the authors pointed out. Significantly, exposing rat neurons to ceramides resulted in the same mitochondrial elongation as had exposure to the progressive MS CSF (Figure 4).
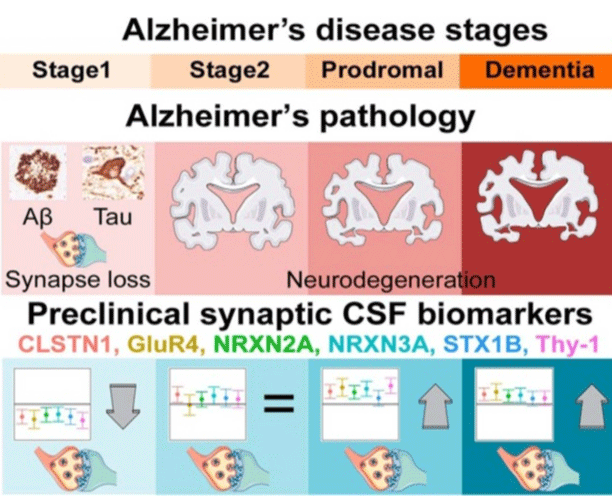
Figure 4: Alzheimer disease stages.
When we exposed cultured neurons to ceramides, we elicited the same changes caused by exposure to CSF from progressive MS patients,” said Maureen Wentling, PhD a research associate in the Casaccia lab and the study’s first author. Further studies in cultured neurons exposed to ceramide either in conditions of low or high glucose indicated that the treatment impairs ATP production. “… The presence of ceramides interferes with the activity of respiratory chain complexes which become dysfunctional,” the investigators stated. “The neuron attempts to compensate this energetic deficit by upregulating glucose transporters and re-directing the energetic response towards glycolysis in an attempt to meet the metabolic demand, which in the long terms proves to be inefficient and lead to neurotoxicity. We further discovered that ceramides induced neuronal damage by acting on 2 cellular mechanisms” Wentling added. “On one end, ceramides impaired the ability of neurons to make energy by directly damaging the mitochondria. On the other end, they also forced neurons to more rapidly uptake glucose in an attempt to provide energy for the cell” The neurotoxic effects of CSF on cultured neurons could be reduced by supplementing the neurons with glucose or lactate. Although this approach wouldn’t work as a sustainable therapeutic strategy, the results may help researchers develop new approaches to protect mitochondria in patients with progressive MS, while ceramides in CSF may represent potential biomarkers of neuro-degeneration. “These data suggest a condition of ‘virtual hypoglycosis’ induced by the CSF of progressive patients in cultured neurons and suggest a critical temporal window of intervention for the rescue of the metabolic impairment of neuronal bioenergetics underlying neuro-degeneration in MS patients”. “The role of specific CSF ceramides as potential biomarkers for neuro-degeneration is also of great interest and awaits further validation in larger cohorts of MS patients, in future studies”.
Persistent HIV in central nervous system linked to cognitive impairment
Many people with HIV on antiretroviral therapy (ART) have viral genetic material in the cells of their cerebro-spinal fluid (CSF), and these individuals are more likely to experience memory and concentration problems, according to new data published online today in the Journal of Clinical Investigation. A study of 69 individuals on long-term ART found that nearly half of the participants had persistent HIV in cells in their CSF, and 30% of this subset experienced neurocognitive difficulties. These findings suggest that HIV can persist in the nervous system even when the virus is suppressed in a patient’s blood with medication. The study was funded by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Mental Health (NIMH), both parts of the National Institutes of Health. Investigators from the University N. Carolina, and of Pittsburgh, and Yale Univ. studied participants enrolled in the AIDS Clinical Trials Group (ACTG) HIV Reservoirs Cohort Study. This primarily male group--aged 45 to 56--of long-term HIV survivors had infections controlled with ART for on average nine years. Researchers analyzed each participant’s CSF for HIV DNA and then compared these data to each participants’ results from standard neurocognitive evaluations. About half of participants had viral DNA in cells in the CSF, indicating the presence of latent virus, even though standard HIV RNA ‘viral load’ tests of the cell-free CSF fluid were positive in only 4% of participants. Investigators also found that 30% of individuals with persistent HIV DNA in the CSF experienced clinical neurocognitive impairment compared with 11% of individuals whose CSF did not contain viral DNA. Many researchers hypothesize that HIV-related inflammation cause’s HIV-associated neurocognitive disorder (HAND). The new findings suggest that the presence of persistent HIV-infected cells in the central nervous system (CNS), despite long-term ART, may play a role in neurocognitive impairment (Figures 5,6). The authors note that the overall frequency of neurocognitive impairment in this group was relatively low and that the association does not confirm that HIV DNA causes HAND. The current study found that examining CSF cells revealed a higher-than-expected prevalence of persistent HIV in the CNS, which may be a significant obstacle to efforts to eradicate HIV from the body.”
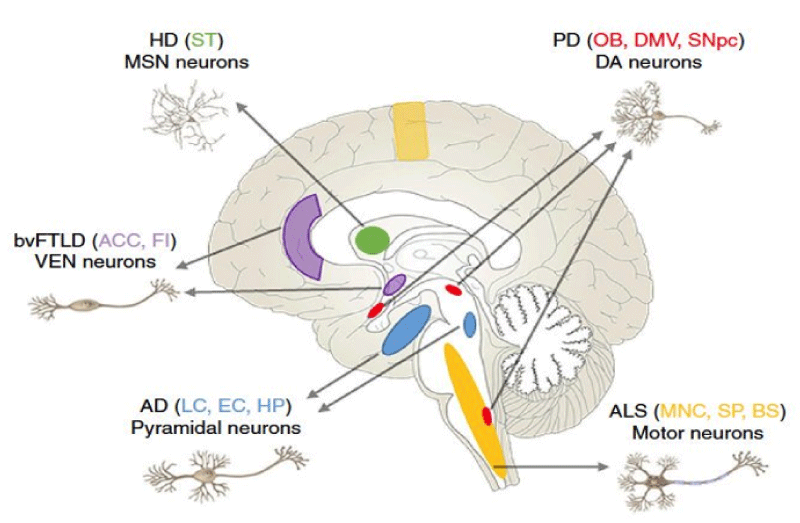
Figure 5: Regions and Neurons vulnerability in neurodegenerative pathology.
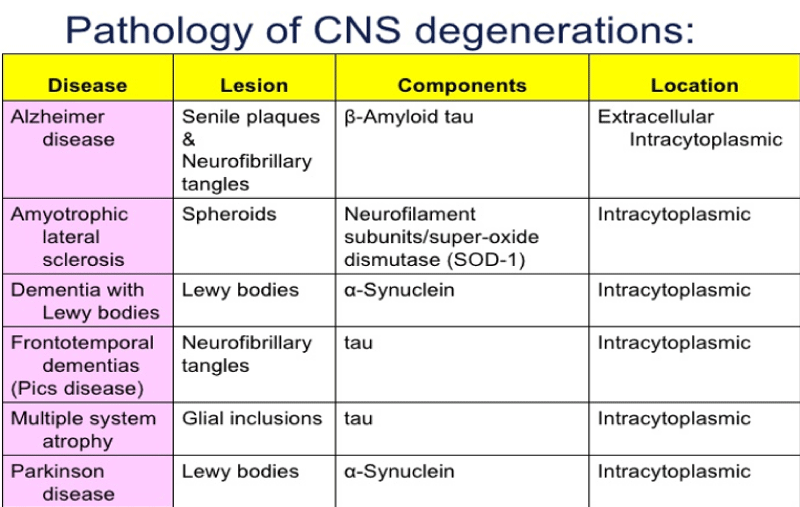
Figure 6: Pathology of CNS degenerations.
New method to gather CSF samples in mice aids in study of sanfilippo
Scientists have developed a new method to gather samples of cerebro-spinal fluid (CSF) - the liquid that circulates in the brain and spinal cord — in mice that allows them to analyze levels of a specific biomarker for Sanfilippo syndrome. The study, “Collection of cerebro-spinal fluid from murine lateral ventricles for biomarker determination in mucopolysaccharidosis type IIIA,” was published in the Journal of Neuroscience Methods. Sanfilippo syndrome, also known as mucoplysaccharydosis III (MPS III), is a lysosomal storage disorder (LSD) caused by mutations in the SGSH gene, which provides instructions for the production of an enzyme called sulfamidase. This enzyme is responsible for breaking down large sugar molecules called heparan sulfate. MPSIIIA, one of the four subtypes of Sanfilippo syndrome, is the most prevalent and severe form of the disease. A mouse model of MPS IIIA, which faithfully recapitulates the human disease, has provided a platform for the testing of treatment strategies, including enzyme replacement therapy and gene therapy. In these preclinical studies, CSF collection could have provided a platform for measuring biomarkers for biochemical assessment of therapeutic efficacy that is translatable to human patients in which collection of brain tissue is not possible,” the investigators said. Collecting pure samples of CSF from animals with sufficient volume to measure the levels of specific disease biomarkers has proven to be a challenge for researchers. Now, a group of researchers from the University of Adelaide in Australia developed a method that allows them to gather pure CSF samples from the brains of adult mice to measure levels of a biomarker of MPSIIIA. During the assay, animals are anesthetized and placed in a stereotactic device, used to perform minimally invasive brain surgery. A small needle is inserted into the brain’s lateral ventricles — cavities in the brain that are filled with CSF — and a micro-syringe pump is used to extract the samples.
With the new method, researchers were able to collect up to 10 μL of pure CSF that was clear and had the consistency of water. Moreover, they found this small amount of fluid was sufficient to measure levels of heparan sulphate disaccharidea (a disease biomarker of MPSIIIA, HNS-UA) and allow them to distinguish healthy animals (levels lower than 5 pmol/mL) from MPSIIIA mice (average of 143 pmol/mL). “Advantages of this method over the most commonly used … collection technique include increased CSF sample volume (10 μL) and reduced blood contamination. One drawback of CSF collection via the lateral ventricles was the time taken for collection,” the researchers said. “In conclusion, we report an alternate method for the reliable collection of pure CSF samples from mice and show that a disease-specific biomarker can be quantified. By including CSF assessment in mouse studies that aim to elucidate neuropathological mechanisms and/or assess potential therapies, a translation into patients, from which only CSF can be collected, is possible,” they said.https://www.prnewswire.com/news-releases/cerebro-spinal-fluid-csf-management-market-is-estimated-to-reach-us1-84-billion-due-to-increased-healthcare-spending-worldwide---tmr-300869353.html. The global cerebro-spinal fluid management market was estimated to be worth US$1.13 bn in 2014. The market is anticipated to rise at a healthy CAGR of 5.5% over the forecast tenure 2015 to 2023. At this rate, market revenue is estimated to reach US$1.84 by 2023-end. CSF shunts were most demanded in 2014 and are anticipated to continue to be the dominant segment during the forecast period. The CSF drainage system segment, is also planned for the highest CAGR on the market. Most CSF leadership demand was supported by North America in 2014 and it is likely to stay the most profitable region during the forecast period. Increased Government Funding Supporting Global CSF Management Market Growth.
The world is home to 841 million individuals aged 60 or older, according to the United Nations (US). By the end of 2050 the UN anticipates that the figure will exceed 2 billion. Because geriatrics are usually neurologically affected by such illnesses as Alzheimer’s and Parkinson’s, this exponential increase is primarily responsible for the increasing demand in the CSF market. The study also points to a general increase in the incidence of neurological diseases, increasing government and personal financing, and the growing demand for minimally invasive operations (Figure 7). These are some of the other factors that will aid the growth of the global cerebro-spinal fluid management market in coming years. The world’s rapidly developing countries such as China, India, and Brazil are expected to hold strong potential for the CSF management market in future. Presence of vast populations and rising disposable income of the urban populace in these regions enhances their purchasing power. These people thus end up spending a lot more on healthcare, which in turn fuels the CSF management market.
Figure 7: LCR flux.
Unmet clinical needs to fuel cerebro-spinal fluid management market growth
The growth in this industry will increase over the forecast period through the development of platforms for the identification and development of proprietary techniques to resolve unmet clinical need and treat chronic nervous diseases such as hemorrhage Subarachnoid and hydrocefalia. Continuous studies undertaken on the identification and development of CSF platforms in neurotoxic disease treatments has been attributed to a significant boost to industry development. It is expected that the application of such platforms will help to reduce the use of hospital funds and improve clinical functional results by accelerating the adoption by reducing the duration of their stay. This is expected to be a promising avenue for vendors in the global cerebro-spinal fluid management market in coming years. The global cerebro-spinal fluid management market is predicted to witness lucrative growth in coming years, according to research by Transparency Market Research (TMR). Comprising of a large pool of vendors, the global cerebro-spinal fluid (CSF) management market fosters high competition. Vendors in the market are leveraging novel growth strategies in order to gain momentum in the industry. Vendors in the global CSF management market are engaged in mergers and acquisitions, product development, regional expansion, and collaboration. For instance, Codman Neuro recently introduced an MRI-resistant programmable valve that offers a range of pressure settings, including CSF drainage and intra-ventricular pressure for treatment of hydrocephalus. Medtronic Plc. announced StrataMR valve and shunts clearance by the United States Administration of Food and Medicines (FDA). This complements the Strata Adjustable Valve Systems portfolio of Medtronics, which treat hydrocephalus and cerebro-spinal fluid disorders patients. Medtronic’s endorsement will assist finish its portfolio of full-body MRI access technologies like pacemakers, DBS, cardioverters (ICDs), and spinal cord stimulators. Specifically, this will assist Medtronic in finishing its portfolio of technologies. Such developments are likely to amplify competition in the global cerebro-spinal fluid management market in coming years. This review is based on TMR’s report CSF Management Market (Product - CSF Shunts and CSF Drainage Systems) - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2015 - 2023.”
Sense of smell, pollution and neurological disease connection explored
A consensus is building that air pollution can cause neurological diseases such as Alzheimer’s disease and Parkinson’s disease, but how fine, sooty particles cause problems in the brain is still an unanswered question. Now a team of Penn State researchers, using mice, have found a possible way, but more research is still needed. “The researchers looked at how cerebro-spinal fluid, the liquid that flows around the brain and spinal cord, flows out through the nose, and what happens when the flow of fluid is stopped. “There has been a lot of interest in understanding cerebro-spinal fluid movement in the last 5 years,” said P. Drew, Huck Ass. Prof. of Engineering Science and Mechanics, Neurosurgery and Biomedical Engineering. “More and more it is realized that it does not just cushion the brain, but may also transfer stuff out of the brain and spinal column area.” The question is how the cerebro-spinal fluid-or CSF does — leave the enclosed area of the brain and spinal column and where does it go? Research into old scientific papers indicated that some scientists had speculated that one exit pathway was through the nose. “I was trying to label cerebro-spinal fluid with a dye for another experiment,” said Jordan N. Norwood, graduate student in cellular and developmental biology and Drew’s student. “We started seeing this dyed cerebro-spinal fluid drain out through the nose.” More research into old scientific papers showed that not only had others suggested that the cerebro-spinal fluid left through the nose, but that there was a connection to the sense of smell. The researchers also found that there is a long-held connection between loss of smell and the early beginnings of such neurological diseases as Alzheimer’s disease and Parkinson’s disease. Using chemical ablation, the researchers destroyed the olfactory sensory nerves that come through the mouse’s hard palate. Destruction of these nerves causes loss of the sense of smell, but also caused the flow of cerebro-spinal fluid to stop. “The mice seem normal after we used zinc sulfate to ablate the nerves in the nose,” said Drew. Because the flow of fluid from the nose stopped, the researchers checked to see if the pressure around the brain and in the spinal cord increased. “Animals and people are constantly making CSF so if it doesn’t go out, pressure will go up,” said Drew. “But we found that the pressure did not increase after the flow from the nose stopped.”
The researchers believe that some other pathway may increase its flow or CSF to compensate for what would normally go out through the nose. These other pathways could include those around the brain that drain into the lymphatic system. Another possibility is that the production of CSF decreases in response to stoppage of CSF flow through the nose. The researchers suggest in a recent issue of eLife, “that damage to olfactory sensory neurons (such as from air pollution) could contribute to altered CSF turnover and flow, providing a potential mechanism for neurological disease.” They also state that “reduced CSF turnover may be a contributing factor to the buildup of toxic metabolites and proteins that cause neuro-degenerative disorders.” Both the effects of pollution and the effects of reduced CSF turnover might explain the origin of some of these diseases. “By ablating the neurons, we were able to disrupt and disable flow in the nose,” said Norwood. “People in areas with heavy air pollution may be breathing stuff that does the same thing as our experiments. Next we would like to collaborate with a lab in the Materials Research Institute that is working with soot or jet fuel particles to see if we get the same effect,” she added.”
Detoxing For brain health - new research findings
CranioSacral Therapy Improves Glymphatic Cleansing of Brain Tissue. Carolyn Simon: “New research provides evidence the body has a fast-track brain cleansing system that prevents diseases such as Alzheimer’s and maintains brain health. Finding ways to support and enhance this cleansing process could lead to improved outcomes in brain injury and brain disease. Read on to find what scientists have discovered and how cranio-sacral therapy effectively promotes brain health by invigorating this active fluid cleansing system. Most of us have heard of the lymphatic system, the collection of vessels and nodes running throughout the body that helps cleanse waste products and is part of the body’s immune system. Now a team of neuroscientists at the University of Rochester Medical Center has identified a fascinating fast-track cleansing system in the brain called the glymphatic system. Their findings, published online in 2012 issue of Science Translational Medicine, were only possible using the new technology of 2-photon microscopy. This allows researchers to study and track the flow of blood, cerebro-spinal fluid (CSF) and other substances in live brain tissue. The glymphatic pathways only operate in a living brain, so they were not scientifically observable until now.
Scientists named it the glymphatic system because it functions much like the lymphatic system but is managed by the glial cells within the brain. Glial cells are non-neuronal brain cells with several regulatory and protective roles including destruction of pathogens and removal of dead nerve cells. What the 2-photon microscope shows is glymphatic pathways circulating CSF efficiently throughout every part of the brain, along specialised anatomical structures. Previously scientists hypothesised that CSF slowly trickled through brain tissue and filtered out waste material gradually, but this is only part of the picture. Now we know the glymphatic system is pushing large volumes of CSF very quickly and very deeply into the brain, much faster than was previously thought, to transport waste away under pressure. Specifically, a bulk flow process moves CSF via the arterial system right into the brain tissue, exchanging with the interstitial fluid inside the brain. As it does, it washes through the tissue collecting waste particles that are sitting in between the brain cells. The CSF then enters the venous system via veins within the brain tissue, taking the fluid and the waste it picks up away from the brain. In this way waste material is efficiently removed from the brain tissue, by the CSF, via the circulatory system.
We know accumulation of waste and toxic matter in the brain environment adversely affects brain function. The discovery of the glymphatic system opens up the research field. Neuroscientist Maiken Nedergaard said, “We’re hopeful that these findings have implications for many conditions that involve the brain, such as traumatic brain injury, Alzheimer’s disease, stroke, and Parkinson’s disease”. The focus is finding how the glymphatic system might be implicated in cause and/or recovery. The Glymphatic System and Alzheimer’s Disease One of the first extracellular waste products researched in the context of Glymphatic cleansing was amyloid β. Amyloid β is a protein made and secreted by the brain cells in an on-going process and used to perform several regulatory and protective functions. Because the brain is continuously producing this molecule it needs to clear out any amyloid β it’s no longer using. In AD the amyloid β builds up in the brain, clogging up the spaces in between the cells. Researchers think it’s these amyloid β plaques that kill the neurons and cause the dementia that is a primary symptom of Alzheimer’s. In Alzheimer’s the glymphatic cleansing pathway may be failing, causing the increasing deposition of amyloid β. The fast-track cleansing pathway may have stopped working properly due to deterioration through ageing processes, or the effect of a previous infection or injury.
It may be possible to slow the progression of Alzheimer’s by increasing the rate of flow of the glymphatic system, thereby flushing the amyloid β out more quickly. Where deposits of amyloid β have accumulated, improving the glymphatic flow and its cleansing effect could break down and reduce these deposits and clear them more quickly from the brain via the circulatory system. The good news is we already have an effective therapy for improving glymphatic flow. Cranio Sacral Therapy Enhances Glymphatic cleansing although glymphatic cleansing is a newly identified process, the concept of a stronger fluid motion through the brain is not new. Craniosacral therapy pioneer Dr. John Upledger hypothesised his “Pressurestat Model” of fluctuating CSF production within a semi-closed hydraulic system back in the early 1980s. This model of CSF moving under pressure within the dural membranes surrounding the brain and spinal cord was the basis of his evolving research and development of craniosacral therapy. There is now an extensive body of evidence of the health-promoting effects of craniosacral therapy, published by craniosacral therapists among a worldwide network of practicing clinicians. Craniosacral therapy is a gentle, hands-on body therapy that engages with the body’s craniosacral system, the interactive physiological environment surrounding and protecting the brain and spinal cord (Figure 8). The focus in craniosacral therapy is encouraging the release of trauma locked within the tissues, improving physiological function and promoting the body’s natural healing processes. Craniosacral techniques restore and enhance fluid movement within the brain and spinal cord and throughout the whole body. During craniosacral therapy cerebro-spinal fluid motion is increased, improving glymphatic flushing of the brain tissues. Adequate flushing of the brain environment is essential for brain detoxification, nutrition and normal range of function. Scientists’ recent discovery of the glymphatic system’s mechanism informs Dr. Upledger’s earlier hypothesis. Just as importantly, it affirms craniosacral therapy as an effective and established treatment option for enhancing brain cleansing in cases of brain disease or injury and as a preventative measure.”
Figure 8: Dementia.
Brain’s drain: neuroscientists discover cranial cleansing system
“Fluids coursing through the nervous system could help clear the brain of toxic detritus that leads to Alzheimer’s and Huntington’s disorders 2012. The brain can be a messy place. Thankfully, it has good plumbing: Scientists have just discovered a cleansing river inside the brain, a fluid stream that might be enlisted to flush away the buildup of proteins associated with Alzheimer’s, Huntington’s and other neuro-degenerative disorders. The researchers, based at the University of Rochester (U.R.), University of Oslo and Stony Brook University, describe this new system in the journal Science Translational Medicine today. The study adds to the evidence that the star-shaped cells called astrocytes play a leading role in keeping the nervous system in good working order. In most of the body, a network of vessels carry lymph, a fluid that removes excess plasma, dead blood cells, debris and other waste. But the brain is different. Instead of lymph, the brain is bathed in cerebro-spinal fluid. For decades, neuroscientists have assumed that this fluid simply carries soluble waste by slowly diffusing through tissues, then shipping its cargo out of the nervous system and eventually into the body’s bloodstream. Determining what’s really going on has been impossible until recently. In this study, researchers led by U.R. neuroscientist M. Nedergaard have identified a second, faster brain-cleansing system. Nedergaard an expert in non-neuronal brain cells called glia, has long suspected that these cells might play a role in brain cleansing. Nedergaard and colleagues studied live mice with holes drilled into their skulls to gain an unobstructed view. To see how waste is carried by cerebro-spinal fluid in a living mouse, they injected the mice with radioactive molecules that could be traced using laser-scanning technology.
The molecules’ journey began after being injected into the subarachnoid space, a cavity between membranes covering the brain and spinal cord. The researchers observed that, like a river, cerebro-spinal fluid carried these molecules rapidly along specific channels. Glial cells along the outside of arteries form these channels, creating a flume for cerebro-spinal fluid that follows the brain’s blood vessels. In addition, the researchers found that these glial cells mediate the channel’s activity, assisting the flow of fluid through the channel. From channels alongside arteries, the tracer-bearing fluid then passes through brain tissues. At the other end of tissues, it flows into similar channels along veins. The fluid follows these veins then either returns to the subarachnoid space, enters the bloodstream or eventually drains into the body’s lymphatic system. The researchers christened the network the “glymphatic” system, a nod to both glial cells and its functional similarity to the lymphatic system. U.R. neuroscientist and lead author Jeff Iliff notes several surprises in the study: “I didn’t think we would see these jets of fluid going through the brain,” Iliff says.he explains that previous conception of cerebro-spinal fluid’s role in waste removal suggested that the process was one-way, sending particle-carrying fluid from the brain into the body. Instead, they observed a recycling, as much as 40 percent of the fluid returned to the brain. As a test of their work, the researchers injected proteins called amyloid beta into mice’s brains. In Alzheimer’s, this protein—present in all healthy brains—can accumulate and clump, developing into cell-damaging plaque. The researchers compared mice with a normal glymphatic system to those with a disabled gene that prevented glial cells from assisting in the fluid flow. They found that in the normal mice, the protein rapidly cleared from the brain along these channels, but amyloid removal diminished in the gene-altered animals (Figure 9). Iliff hypothesizes that a faulty glymphatic system may bear the blame for the over-accumulation of proteins seen in Alzheimer’s, amyotrophic lateral sclerosis, Huntington’s and other neuro-degenerative disorders—and further study may even reveal a way to dispose of these clumps. Jaleel Miyan, a neurobiologist at the Univ. of Manchester in England who did not participate in this research, stressed the significance of this finding by characterizing the analogy with the lymphatic system as inadequate: “What they have demonstrated is actually far more extensive and important to CSF biology.” The study clarifies discrepancies in past research and may lead to a better understanding of the functioning of the glymphatic system as a possible cleanser of the neural toxins that inevitably accrete and do damage as we age.”
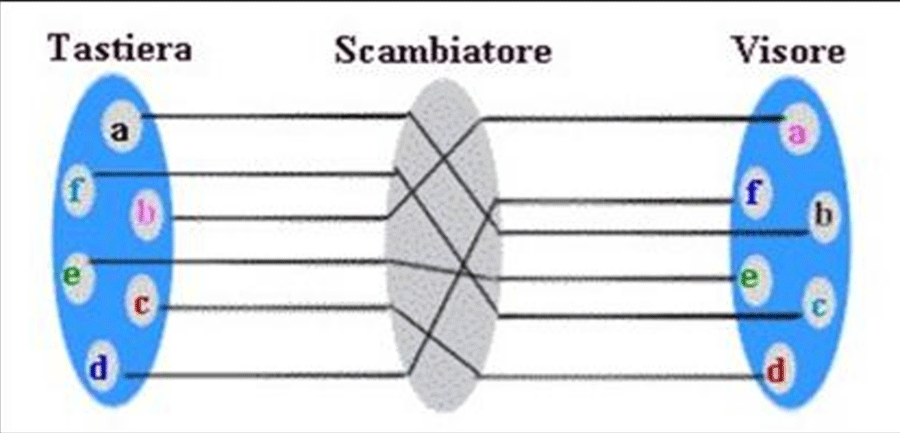
Figure 9: ENIGMA machine (word war second secret way of communication used by Germany).
Brain may flush out toxins during sleep
NIH-funded study suggests sleep clears brain of damaging molecules associated with neuro-degeneration. A good night’s rest may literally clear the mind. Using mice, researchers showed for the first time that the space between brain cells may increase during sleep, allowing the brain to flush out toxins that build up during waking hours. These results suggest a new role for sleep in health and disease. The study was funded by the National Institute of Neurological Disorders and Stroke (NINDS), part of the NIH.” “Sleep changes the cellular structure of the brain. It appears to be a completely different state,” said Maiken Nedergaard, M.D., D.M.Sc., co-director of the Center for Translational Neuromedicine at the University of Rochester Medical Center in New York, and a leader of the study. For centuries, scientists and philosophers have wondered why people sleep and how it affects the brain. Only recently have scientists shown that sleep is important for storing memories. In this study, Dr. Nedergaard and her colleagues unexpectedly found that sleep may be also be the period when the brain cleanses itself of toxic molecules. Their results, published in Science, show that during sleep a plumbing system called the glymphatic system may open, letting fluid flow rapidly through the brain. Dr. Nedergaard’s lab recently discovered the glymphatic system helps control the flow of cerebro-spinal fluid (CSF), a clear iquid surrounding the brain and spinal cord. After this first refrence reported: Observing other science like informatics and the translation of information by algorytm is possible to have new powerful tools to better treat the neuro-degenerative disease we have seen: Turing machine (1936) is a mathematical- model of computation that defines an abstract machine, that manipulates symbols on a strip of tape according to a table of definite rules.
Algorithm: simulating that algorithm’s logic can be constructed by A Turing
A Turing machine is an example of a CPU that controls all data manipulation done by a computer, with the canonical machine using sequential memory to store data. It is a machine capable of enumerating some arbitrary subset of valid strings of an alphabet; these strings are part of a recursively enumerable set. A Turing machine has a tape of infinite length on which it can perform read and write operations. In project named ULTRA A. (In Second World War) A. Turing make possible to translate with an algoritm the secret messages produced with ENIGMA.
INPUT ----------------OUTPUT
In some medical condition to reduce the toxicity os some metabolic product are used specific procedure (depurative) in order to reduce toxic level (in example in blood). But can we think to apply this general concept to other not classic situation like some spinal cord neuro -degenerative condition like some ALS forms involving SOD disfunctions or brain conditions like DA and other?
TOXIC-MICRO-ENVIRONMENT---------DETOXICANT PROCEDURE
NORMAL VELOCITY OF DISEASE PROGRESSION-----DELAY
Methods: medical devices, depurative procedure, persistance of actions, Level of efficacy
Principles: The procedure must efficacy remove the toxic catabolic methabolic- pathological substantia (using or not a pharmacological - or other phisic prcess that increase the efficacy in remove process).
Constraints: No added toxicity or damage to the tissue
Forced diuresys, Urine alcalinization: In some in poisoning also other antidothes use: COMPLEXANT agents for some heavy metal poison, Cyanide binder and other. In all this procedure a toxic substantia is removed from a body part with low damage for the organism.
Whit an observational approach some relevant literature (in our opinion) is analyzed in order to produce a global hypothesis of work to be submitted to the researcher. All literature comes from biomedical databases (like PUBMED). After this review process a practical experimental project hypotesys (in vitro) is provided.
According to article: Amyotrophic Lateral sclerosis and endogenous-esogenous toxicological movens: New model to verify other pharmacological strategies
Related the body region of onset, a mix of upper and lower motoneuron deficits and rate of progression. The endogenous neuro- microenvironment in determinate genetic profile is heavily involved with the neuronal damages and strategies that can control or modify it can be useful in preventing the progression of some neuro chronic degenerative- disease. An exogenous or endogenous toxicology approach (similar to an antidothes approach or a depurative strategies) added to the best new pharmaceutical instrument can be a way to be run to protect the motor-neuron from a poison like process. The cell death due by apoptosis (free radicals, excitotoxicity, flogosis, immune reactions, toxic exogenous substances and other can be avoided or reduced introducing new depurative strategies (against TOXIC-X Or dangerous micro- or local environment) or other Technique’s to shift the oxidative damage from the motoneuron to other substances (or other physic procedure, medical devices and other artificial implants to improve global activity). If considered like a VECTORS in Physical science the two vectors: intrinsic neuronal weakness and the exogenous endogenous toxicologic substances moves in the same direction: a moto neuron damage (Figure 10). Related to this conclusion new pharmacological strategies or high improving in local availability of therapeutic substations can improve clinical outcomes by clinicians (Figure 11). Better efficiency in pharmaco-kinetics, BEE pass level, persistence of action, low local toxicity, and other properties, medical devices use, innovative Nano drug delivery systems, alternative way of sub ministration). A Medicinal chemistry- pharmaceutical and toxicological approach can be the right instrument to be added to the actual therapeutic scenario of this neuro-degenerative disease (better pharmaco-kinetics in BEE pass can be relevant also in other field like oncology- ematology), An interesting example comes from other different field but that can be useful to our experimental project cars body parts protection in the beginning of the 20th century. When steel was rusting chemist-oriented scientists came up with an idea to include a small portion of zinc, as the coding these steel parts, as Zinc has more tendency to oxidize than iron, so the oxidation would be diverted to zinc, instead of iron. Could we take the same principle and apply to some sort of drug or other artificial system, which can do the same to the nerves? So, to speak we want some-thing to stop the radical chain of the damage inflicted upon the defenseless nerve so something else takes the damage away from the nerve. The pharmaceutical scientists suggested the use of antioxidants such as Vitamin C, vit E and other, but showing un-satisfiable response. In other words, maybe there is no enough vitamin C or other antioxidants sustained at the target?
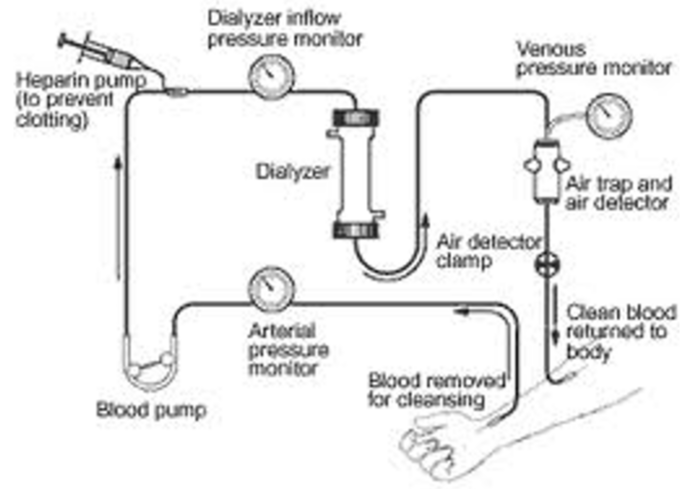
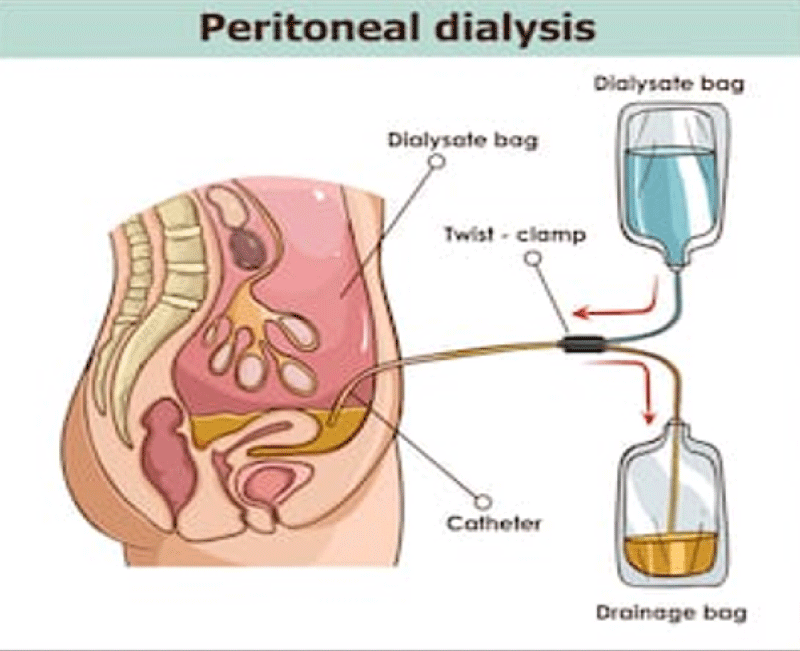
Figure 10: Dyalisis scheme.
Figure 11: Peritoneal dialisy, Emoperfusion, Emofiltration plasmaferesys.
So since after each administration, the drug levels at the target fall immediately or in some cases in a short span of time would a sustained release in a loco-regional fashion can recompense this process of loss of activity? That’s assuming that the trouble makers are free radicals. But regardless, once we can find a technology that can deliver site-specifically the drug to the tissue around the nerve around the part of the body that specific location in which say for example lumbar the damage is happening, of course with an extended release feature, instead of antioxidants, we may use other drugs, including corticosteroids or another molecule? So, we believe there are 2 problems: one is that we don’t exactly know with what mechanism this phlogosis damage is happening, but more importantly we certainly have also a pharmaco-kinetic and biodistribution problem [1]. And in article Role of plants, environmental toxins and physical neurotoxicological factors in Amyotrophic lateral sclerosis, A D DA and other Neuro-degenerative Diseases 2018 “ALS is a chronic disease and so is related to the progressive damage time related so is possible to think to a in vitro model that compare normal nerve tissue in First fase of disease with other normal nerve tissue not involved in this kind of pathology and observing if local micro-environment contributes in high way to the progression. The same verify this condition in first phases and then advanced phases of disease (progression). In this way is possible to confirm or not soluble factors involved (Figure 12). The same also using the same liquid medium we can observe if present an intrinsic weakness of the nerve tissue affected vs normal tissue. In this experimental project we can show even intrinsic weakness of neurons but also the activity of a soluble factor:
Figure 12: Ferrous rust.
In example 4: in vitro experimental sample (animal model):
a) Neurons Sla affected + Liquid medium of affected tissue
b) Neurons Sla affected + Normal liquid medium
c) Neurons not Sla affected + liquid medium affected tissue
d) Neurons not Sla affected + normal liquid medium is interesting verify ALSO if using a medical device system is possible to get a more persistence of activity of the measure adopted (pharmacological depurative- detoxicant or with other mechanism that can improve the persistence of action in local spinal cord)” [2].
According to article: endogenus toxicology: modern physio- pathological aspects and relationship with new therapeutic strategies. an integrative discipline incorporating concepts from different research discipline like biochemistry, oharmacology and toxicology
A new scientific discipline named ENDOGENOUS TOXICOLOGY must be introduced as a useful instrument to better clear some pathological process and also to introduce better and new pharmacological (and not) strategies.IT is relevant is to consider some pathology under a toxicological aspect and endogenous process time related. (Topographic condition, time of exposure, catabolic status). A better knowledge related basic pathologic process make possible to verify and introduce new therapeutic strategies to achieve better global clinical results. Concepts from toxicological sciences like dosage, time of exposure, kinetics, metabolism, dynamics and other must be applied also in endogenous toxicological – pathological process. Like classic GENERAL toxicology science concept like:
• Endogenous local Toxic substanties
• Topography of the toxic process
• Metabolism-catabolism of this toxics
• Measure methods
• Kinetics, dynamics
• Dose- response relationship
• Risk factors
• Worsening local endogenous conditions, Additive
Condition
• Preventing strategies
• Depurative methods, inactivating methods- Antidothes approach
• And other must be used also in this new scientific Discipline [3]
Altaf Alabdali, et al: Exposure to even low levels of lead (Pb) early in life has adverse effects on a variety of cognitive and behavioral functions and neurochemical systems, resulting in deficits in learning, memory and attention that may persist into adulthood. Persistent effects of Pb exposure early in life can produce changes that arise from physiological re-programming. In this study, there was a significant increase in Pb levels in patients with ASD compared with the control group, coupled with a correlation between Pb concentration and the severity of SRS and CARS scores. These observations support a recent study on patients with ASD by Schneider et al., who suggested potential epigenetic effects of developmental Pb exposure on DNA methylation. These effects were mediated at least partially through the dysregulation of methyltransferases as multiple forms of proteins, at least some of which are potentially involved in cognition and the resulting abnormalities were recorded in patients with ASD. The reported elevation of Hg and Pb in the RBCs of patients with ASD compared with the control subjects can be related to and support a previous study by Al-Yafee et al. in which Saudi patients with ASD were described as poor detoxifiers with a lower GSH/GSSG ratio and remarkably less active GST and thioredoxin reductase as markers of the detoxification mechanism. It is well known that GSH and GST are both critical for the detoxification of mercury. While GSH carries Hg through biliary transport for excretion, Hg2+ rapidly oxidizes glutathione. This observation is correlated with the previous work of Al-Gadani et al. who reported lower GSH in the plasma of Saudi patients with ASD. Additionally, the increase of Hg and Pb recorded in this study is consistent with previous studies. For example, Blaurock-Busch et al. found a significant increase of both toxicants in the hair of autistic children compared with non-autistic children. This observation may indicate an impaired detoxification mechanism as a risk factor that significantly contributes to the etiology of autism. The exposure of patients with ASD in early childhood to mercury vapor, methylmercury and ethylmercury may occur through dental amalgams, fish intake and vaccinations.
In this study, remarkably higher levels of Hg and Pb were recorded in patients with severe social and cognition impairment compared with those with mild-moderate abnormalities. This observation suggests that heavy metal toxicity is closely related to the pathophysiology of autism. This outcome does not concur with a study by Elsheshtawy et al. in which Hg, but not Pb, was found in the hair of autistic patients as a biochemical correlate to disease severity. Our results are consistent with the recent work of Adams et al. who reported that children with autism have higher average levels of several toxic metals, among which Hg and Pb are strongly associated with variations in the severity of the disorder. In their study, Adams et al. found a non-significant difference of Hg in children with autism vs. neuro-typical children. This could be attributed to differences in geographic exposure to mercury (Saudi Arabia vs. Arizona). Patients with autism are poor detoxifiers (i.e., unable to detoxify mercury when it reaches a certain level). This could indicate a higher rate of exposure to Hg in the Saudi population compared with the population in Arizona. Face recognition is a core deficit of social impairment in autism. A number of studies indicate that norepinephrine and dopamine modulate and reduce behavioral responses to changes in the social environment. In addition, serotonin transporter binding appears to be reduced in certain brain regions known to play an important role in social cognition and behavior and 5HT binding potential is negatively correlated with social impairment. Therefore, recording Pb as a correlate to severity of SRS and CARS scores in the present study is consistent with the recent findings of El-Ansary et al. in which a positive association was observed between chronic Pb toxicity and lower levels of neurotransmitters as markers of neurologic injury in autistic brains in a Saudi population. Alternately, the biochemical correlation between Pb and the severity of autism is in agreement with other studies. Szkup-Jabłońska et al. reported a significant correlation between Pb and fear, nervousness, verbal and nonverbal communication, social activity level, and consistency of intellectual response. Moreover, the positive correlation between elevated Pb toxicity and both autistic scales could be supported by the fact that Pb exposure affects multiple health outcomes and physiological systems. These include behavioral/cognitive/IQ effects, nerve conductive effects, hearing loss, reproduction and development effects and death from encephalopathy. Long-term trends in population exposure to Pb (indexed through use of leaded petrol and paint) were remarkably consistent with the link between IQ and social behavior.
The significant reduction in plasma GST in Saudi patients with autism compared with controls is documented in this study. This could be related to the significant depletion of GSH as a substrate of GST in the plasma of patients with ASD compared with control subjects. The reduction in this essential detoxifying enzyme can explain the poor detoxification in patients with ASD, leading to the Hg and Pb toxicity discussed above. In our study, there was an inverse relationship between decreased levels of plasma GST and the severity of autism, as measured by the SRS and CARS. Severely autistic cases had a remarkably lower GST activity compared to mild-moderate cases of autism. These outcomes concur with Geier et al, who also found a significant inverse relationship between blood GSH levels and autism severity measured with the CARS. Mercury aggravates impaired glutathione synthesis by depleting glutathione in lymphocytes and monocytes, leading to an increased risk of immuno and cytotoxic effects. Although, the roles and importance of various forms of vitamin E are still unclear, it has been suggested that the most important function of α-tocopherol is as a signaling molecule playing an important role in protecting neurons from damage. As an antioxidant, vitamin E may prevent or reduce the propagation of free radicals, which are associated with physical decline, in the human body. This may help reduce muscle or DNA damage and prevent the development of pathological conditions, such as autism. Herndon et al. also found decreased vitamin E levels in autistic patients. The brain contains high levels of oxidizable lipids that must be protected by antioxidants; hence, the supplementation of ASD patients with vitamin E as a major lipophilic antioxidant could be helpful. The highly significant correlation between vitamin E depletion and severity of autism, as measured by the SRS and CARS, supports its critical role in protecting against the toxic effects of Pb and Hg. This is consistent with a previous report by Adams et al. that showed a significant association between vitamin E insufficiency and the severity of the Autism Scale (SAS).
All measured parameters demonstrated almost 100% sensitivity and very high specificity, which also confirmed the hypothesis that autistic patients are poor detoxifiers, unable to readily excrete toxic substances (Hg and Pb), and suggests that reduced GST activity and depleted vitamin E are two critical factors related to poor detoxification. Lead, Hg, GST and vitamin E show perfect predictiveness curves. Excellent predictiveness curves for the four parameters reflect the possibility of using any of these parameters to follow up an antioxidant-related treatment strategy. A successful treatment could be followed through a remarkable elevation of plasma vitamin E, the activation of GST or both in autistic patients. Alternately, efficacious treatment could occur through a reduction in Pb and Hg levels. In addition, the relationship between vitamin E deficiency and the etiology of autism could be ascertained by the high specificity, sensitivity and AUC, as shown with the ROC analysis. The negative correlations between Hg & Pb and vitamin E & GST suggest the use of vitamin E as a non-enzymatic antioxidant in treating patients with autism. This suggestion is supported by the multiple regression analysis results, confirming that higher levels of Hg and Pb, together with lower levels of GST and vitamin E, can be used to predict cognitive and social impairment with the regression of both antioxidant parameters, which is more related to abnormalities of both. The high values of both sensitivity and specificity recorded for Pb, Hg, GST and vitamin E, together with the good predictiveness curves; suggest that these can be used as biomarkers for measuring the severity of SRS and CARS scores in a Saudi population. This study confirmed the impaired antioxidant and detoxification mechanisms in Saudi autistic patients. Hence, early intervention through the supplementation of good quality and safe antioxidants, including vitamin E, carnosine and selenium, can be helpful in decreasing the burden of heavy metal toxicity. Vitamin E exists in eight different forms: four tocopherols and four tocotrienols. The measured form of vitamin E, α –tocopherol, is one of the forms that regulate signal transduction pathways by mechanisms that are independent of its antioxidant properties, and its use as a supplement can be effective in reducing the toxicity burden in these patients. Autistic children who undergo intensive intervention have better social interaction than children who do not” [4].
Maiken Nedergaard, et al: An internal plumbing system rids the brain of toxic wastes. Sleep is when this cleanup ritual occurs. The human brain weighs only about three pounds, or roughly 2 percent of the average adult body mass. Yet its cells consume 20 to 25 percent of the body’s total energy. In the process, inordinate amounts of potentially toxic protein wastes and biological debris are generated. Each day, the adult brain eliminates a quarter of an ounce of worn-out proteins that must be replaced with newly made ones, a figure that translates into the replacement of half a pound of detritus a month and three pounds, the brain’s own weight, over the course of a year. To survive, the brain must have some way of flushing out debris. It is inconceivable that an organ so finely tuned to producing thoughts and actions would lack an efficient waste disposal system. But until quite recently, the brain’s plumbing system remained mysterious in several ways. Questions persisted as to what extent brain cells processed their own wastes or whether they might be transported out of the nervous system for disposal. And why is it that evolution did not seem to have made brains adept at delivering wastes to other organs in the body that are more specialized for removing debris? The liver, after all, is a powerhouse for disposing of or recycling waste products. About five years ago we began trying to clarify how the brain eliminates proteins and other wastes. We also began to explore how interference with that process might cause the cognitive problems encountered in neuro-degenerative disease. We thought that disturbances in waste clearance could contribute to such disorders because the disruption would be expected to lead to the accumulation of protein debris in and around cells. This idea intrigued us because it was already known that such protein clumps, or aggregates, do indeed form in brain cells, most often in association with neuro-degenerative disorders. What is more, it was known that the aggregates could impede the transmission of electrical and chemical signals in the brain and cause irreparable harm. In fact, the pathology of Alzheimer’s, Parkinson’s and other neuro-degenerative diseases of aging can be reproduced in animal models by the forced overproduction of these protein aggregates.
We found an undiscovered system for clearing proteins and other wastes from the brain—and learned that this system is most active during sleep. The need to remove potentially toxic wastes from the brain may, in fact, help explain the mystery of why we sleep and hence retreat from wakefulness for a third of our lives. We fully expect that an understanding of what happens when this system malfunctions will lead us to both new diagnostic techniques and treatments for a host of neurological illnesses. The Power of Sleep. Having demonstrated that the expansion and contraction of the interstitial space during sleep were important to both brain function and protein-waste clearance, we then wanted to test a corollary to this observation: Could sleep deprivation precipitate neuro-degenerative disease? Experiments that we conducted in mice showed that during sleep, the glymphatic system did indeed remove beta-amyloid from the brain with remarkable efficiency: its clearance rate more than doubled with sleep. On the other hand, mice genetically engineered so that they lacked aquaporin-4 water channels in astrocytes demonstrated markedly impaired glymphatic function, clearing 40 percent less beta-amyloid than control animals. The remarkably high percentage of beta-amyloid removed challenged the widely held idea that brain cells break down all their own wastes internally (through degradation processes called ubiquitination and autophagy); now we know that the brain removes a good deal of unwanted proteins whole, sweeping them out for later degradation. These new findings, moreover, seemed to confirm that the sleeping brain exports protein waste, including beta-amyloid, through the glymphatic transport system. Additional support for this thesis came from David M. Holtzman’s group at Washington University in St. Louis, which demonstrated that beta-amyloid concentration in the interstitial space is higher during wakefulness than in sleep and that sleep deprivation aggravates amyloid-plaque formation in mice genetically engineered to accumulate it in excess.
So far these investigations have not moved beyond basic research labs. Drug companies have yet to consider antidementia therapies that would physically remove amyloid and other toxic proteins by washing out the brain with glymphatic fluids. But maybe they should. New strategies are desperately needed for a disease that costs the U.S. health care system $226 billion annually. A number of clinical trials for Alzheimer’s are under way, although no drug in development has yet demonstrated a clear-cut benefit. Stimulating glymphatic flows offers a new approach that is worth investigating. A pharmaceutical that regulates the glymphatic system by increasing the rate of CSF flow during sleep could literally flush amyloid out of the brain. A treatment used for a well-known neurological syndrome provides a clue that this approach might work. Normal-pressure hydrocephalus, an illness typically seen in the elderly, is a form of dementia in which excessive CSF accumulates in the hollow central brain cavities, the cerebral ventricles. When a procedure called lumbar puncture removes the fluid by draining it out, patients often exhibit remarkable improvements in their cognitive abilities. The basis for this observation has long been a mystery. Our research suggests that restoring fluid flows through the glymphatic system might mediate the restoration of cognition in these patients. Even if a new drug is not imminent, knowledge of the glymphatic systems suggests fresh ideas for diagnosing Alzheimer’s and other neurological conditions. A recent study by H. Benveniste has shown that standard magnetic resonance imaging can visualize and quantify the activity of the glymphatic system. The technology may allow tests of glymphatic flow designed to predict disease progression in patients suffering from Alzheimer’s or related dementias or normal-pressure hydrocephalus. It might even foretell the ability of patients with traumatic brain injuries to recover. Most of our studies of the glymphatic system to date have focused on the removal of protein wastes. Yet the glymphatic system may also prove to be a fertile area for gaining a basic understanding of how the brain works. Intriguingly, fluids moving through the glymphatic system may do more than remove wastes; they may deliver various nutrients and other cargo to brain tissue. A new study showed that glymphatic channels deliver glucose to neurons to provide energy. Further studies are now investigating whether white matter, the insulationlike sheathing around neurons’ wirelike extensions, called axons, may rely on the glymphatic system for delivery of both nutrients and materials needed for maintaining the cells’ structural integrity. Such studies promise to elucidate the many unexpected roles of the glymphatic system in the daily life—and nightlife—of the brain [5].
Dringen R, et al: Peroxides are generated continuously in cells that consume oxygen. Among the different peroxides, hydrogen peroxide is the molecule that is formed in highest quantities. Organic hydroperoxides are synthesized as products of cellular metabolism. Generation and disposal of peroxides is a very important process in the human brain, because cells of this organ consume 20% of the oxygen used by the body. To prevent cellular accumulation of peroxides and damage generated by peroxide-derived radicals, brain cells contain efficient antioxidative defense mechanisms that dispose of peroxides and protect against oxidative damage. Cultured brain cells have been used frequently to investigate peroxide metabolism of neural cells. Efficient disposal of exogenous hydrogen peroxide was found for cultured astrocytes, OLIGO-DENDROCYTES, microglial cells, and neurons. Comparison of specific peroxide clearance rates revealed that cultured OLIGO-DENDROCYTES dispose of the peroxide quicker than the other neural cell cultures. Both catalase and the glutathione system contribute to the clearance of hydrogen peroxide by brain cells. For efficient glutathione-dependent reduction of peroxides, neural cells contain glutathione in high concentration and have substantial activity of glutathione peroxidase, glutathione reductase, and enzymes that supply the NADPH required for the glutathione reductase reaction. This article gives an overview on the mechanisms involved in peroxide detoxification in brain cells and on the capacity of the different types of neural cells to dispose of peroxides [6].
Hedok Lee, et al: The glymphatic pathway expedites clearance of waste, including soluble amyloid β (Aβ) from the brain. Transport through this pathway is controlled by the brain’s arousal level because, during sleep or anesthesia, the brain’s interstitial space volume expands (compared with wakefulness), resulting in faster waste removal. Humans, as well as animals, exhibit different body postures during sleep, which may also affect waste removal. Therefore, not only the level of consciousness, but also body posture, might affect CSF–interstitial fluid (ISF) exchange efficiency. We used dynamic-contrast-enhanced MRI and kinetic modeling to quantify CSF-ISF exchange rates in anesthetized rodents’ brains in supine, prone, or lateral positions. To validate the MRI data and to assess specifically the influence of body posture on clearance of Aβ, we used fluorescence microscopy and radioactive tracers, respectively. The analysis showed that glymphatic transport was most efficient in the lateral position compared with the supine or prone positions. In the prone position, in which the rat’s head was in the most upright position (mimicking posture during the awake state), transport was characterized by “retention” of the tracer, slower clearance, and more CSF efflux along larger caliber cervical vessels. The optical imaging and radiotracer studies confirmed that glymphatic transport and Aβ clearance were superior in the lateral and supine positions. We propose that the most popular sleep posture (lateral) has evolved to optimize waste removal during sleep and that posture must be considered in diagnostic imaging procedures developed in the future to assess CSF-ISF transport in humans. The rodent brain removes waste better during sleep or anesthesia compared with the awake state. Animals exhibit different body posture during the awake and sleep states, which might affect the brain’s waste removal efficiency. We investigated the influence of body posture on brainwide transport of inert tracers of anesthetized rodents. The major finding of our study was that waste, including Aβ, removal was most efficient in the lateral position (compared with the prone position), which mimics the natural resting/sleeping position of rodents. Although our finding awaits testing in humans, we speculate that the lateral position during sleep has advantage with regard to the removal of waste products including Aβ, because clinical studies have shown that sleep drives Aβ clearance from the brain [7].
Jiajun Xu, et al: In this study, we investigated whether nuclear factor erythroid 2-related factor 2 (Nrf2) activation in astrocytes contributes to the neuroprotection induced by a single hyperbaric oxygen preconditioning (HBO-PC) against spinal cord ischemia/reperfusion (SCIR) injury. In vivo: At 24 h after a single HBO-PC at 2.5 atmospheres absolute for 90 min, the male ICR mice underwent SCIR injury by aortic cross-clamping surgery and observed for 48 h. HBO-PC significantly improved hindlimb motor function, reduced secondary spinal cord edema, ameliorated the reactivity of spinal motor-evoked potentials, and slowed down the process of apoptosis to exert neuroprotective effects against SCIR injury. At 12 h or 24 h after HBO-PC without aortic cross-clamping surgery, Western blot, enzyme-linked immunosorbent assay, realtime-polymerase chain reaction and double-immunofluorescence staining were used to detect the Nrf2 activity of spinal cord tissue, such as mRNA level, protein content, DNA binding activity, and the expression of downstream gene, such as glutamate-cysteine ligase, γ-glutamyltransferase, multidrug resistance protein 1, which are key proteins for intra-cellular glutathione synthesis and transit. The Nrf2 activity and downstream genes expression were all enhanced in normal spinal cord with HBO-PC. Glutathione content of spinal cord tissue with HBO-PC significantly increased at all-time points after SCIR injury. Moreover, Nrf2 overexpression mainly occurs in astrocytes. In vitro: At 24 h after HBO-PC, the primary spinal astrocyte-neuron co-cultures from ICR mouse pups were subjected to oxygen-glucose deprivation (OGD) for 90 min to simulate the ischemia-reperfusion injury. HBO-PC significantly increased the survival rate of neurons and the glutathione content in culture medium, which was mainly released from asctrocytes. Moreover, the Nrf2 activity and downstream genes expression induced by HBO-PC were mainly enhanced in astrocytes, but not in neurons. In conclusion, our findings demonstrated that spinal cord ischemic tolerance induced by HBO-PC may be mainly related to Nrf2 activation in astrocytes [8].
Hodges GR, et al: We previously reported that rabbits with quadriplegia, after a single intracisternal injection of gentamicin sulfate, have multiple minute lesions in the white matter of the upper cervical spinal cord and the lower medulla oblongata, most marked at the C2 segment, when light microscopy is used to study the histopathologic changes. Using electron microscopy in the present investigations, we have found that 9 hours after injection of gentamicin sulfate there was marked edema of fibrous astrocytes in the soma and processes of glia limitans. Ribosomes were disarranged. Mitochondria were swollen, the matrix density was increased and contained para-crystalline lattice structure, and the cristae were reduced in number. OLIGO-DENDROCYTES displayed hypertrophy, with proliferation of smooth ER, ribosomes, microtubules, Golgi complexes, and lysosomes. A few axons were sub-segmentally swollen due to axoplasmic edema. At 24 hours, the lesions became obvious by light microscopy. Astrocytes were more edematous and OLIGO-DENDROCYTES remained hypertrophic. Myelin sheaths were tumefied by dissociation of myelin lamellae. Axons were edematously swollen. Lysed axons were surrounded by lysed myelin sheaths. At 48 hours, numerous neuro-axonal end bulbs were formed at the lysed end. Wallerian degeneration was also evident. The data suggest that oligodendroglia actively react to detoxify gentamicin, astroglia become severely edematous but survive, and axons and myelin sheaths are lysed in reaction to the toxicity of gentamicin. The unique distribution of gentamicin lesions in the deeper white matter, with sparing of the overlying marginal myelinated fibers, seems to depend primarily upon the distribution of edematous astrocytes, which are most sensitive to this noxious chemical. Since gentamicin is apparently detoxified by oligodendroglia, the regenerative process of axons starts after a short period of axonal lysis [9].
Sundaram RK, et al: Alzheimer’s Disease is caused by the deposition of insoluble and toxic amyloid peptides (Abeta) in the brain leading to memory loss and other associated neuro-degenerative symptoms. To date there is limited treatment options and strategies for treating AD. Studies have shown that clearance of the amyloid plaques from the brain and thus from the blood could be effective in stopping and or delaying the progression of the disease. Small peptides derived from the Abeta-42 sequence, in particular KLVFF, have shown to be effective binders of Abeta peptides and thus could be useful in delaying progression of the disease. We have taken advantage of this property by generating the retro-inverso (RI) version of this peptide, ffvlk, in different formats. We are presenting a new detox gel system using poly ethylene glycol (PEG), polymerized and cross linked with the RI peptides. We hypothesize that detox gel incorporating RI peptides will act like a ‘sink’ to capture the Abeta peptides from the surrounding environment. We tested these detox gels for their ability to capture bio-tinylated Abeta-42 peptides in vitro. The results showed that the detox gels bound Abeta-42 peptides effectively and irreversibly. Gels incorporating the tetramer RI peptide exhibited maximum binding capacity. The detox gel could be a potential candidate for treatment strategies to deplete the brain of toxic amyloid peptides [10].
Marsala M, et al: To permit long-term measurement of time-dependent changes in levels of dialyzable drugs and transmitters in the spinal intrathecal (i.t.) space of the unanesthetized rat, we developed a dialysis catheter for chronic placement. This was accomplished by constructing a loop probe 9 cm in length from 0.3-mm-diameter dialysis tubing that was made impermeable except for the distal loop. This loop catheter was readily inserted through an incision in the cisternal membrane and passed to the lumbar enlargement. The ends of the catheter were then externalized on the top of the head. To permit i.t. injections, an additional i.t. catheter could also be inserted simultaneously by the same route. For dialysis, an external end of the loop catheter was connected to a syringe pump and perfused with artificial CSF (10 microliters/min) and the out flow collected. A series of studies were performed to demonstrate the characteristics and utility of this technique. Stability of resting release: glutamate and glucose concentrations in spinal dialysate showed no significant changes from 3 to 10 days after implantation. Spinal cord ischemia: ischemia induced by aortic occlusion or cardiac arrest evoked a time dependent increase in retrieved glutamate. Spinal cord compression caused a time-dependent glutamate, aspartate and PGE2 increase. Noxious afferent stimulation induced by the injection of formalin into the hindpaw resulted in a rapid and transient increase in dialysate glutamate concentration. Direct activation of spinal excitatory amino acids receptors by i.t. injection of kainic acid (1 microgram) evoked a significant increase in aspartate and taurine. Continuous delivery of spinal opiate (alfentanil) via dialysis resulted in a maintained, concentration dependent elevation in the thermal escape latencies in the un-anesthetized rat. The loop dialysis catheter provides a robust experimental tool for studying time dependent changes in the concentration of diffusible substances in spinal CSF over an extended post-implantation interval and allows comparison of these changes with concurrently assessed behavioral indices [11]. J. Pineal Res. 2010; 49:201–209 Melatonin plus exercise-based neurorehabilitative therapy for spinal cord injury. SCI is damage to the spinal cord that results in a loss of function such as mobility or feeling. The common causes of damage are trauma (car accident, gunshot, falls, etc.) or disease (polio, spinal bifida, Friedreichs ataxia). SCIs can occur at any level of the cord, and they compromise or cause loss of body function which is specifically associated with the segment of the cord that is injured and the severity of the injury. Because the spinal cord acts as the main information pathway between the brain and the rest of the body, a SCI can have significant physiological consequences.
Following SCI in humans, many individuals have residual motor and sensory deficits that impair functional performance and quality of life. Dependence on a wheelchair for mobility and development of neuropathic pain are two of the most limiting and most common impairments after SCI. It is well documented that the pathophysiology of SCI involves a two-step process, with primary and secondary mechanisms. The primary traumatic mechanical injury to the spinal cord causes death of a number of neurons. These events are then exacerbated by a variety of secondary mechanisms including vascular changes, ischemia, vasospasms, hemorrhage and thrombosis, neuro-transmitter (especially glutamate) accumulation, generation of free radicals and nitric oxide (NO), calcium overload, compromised energy metabolism and inflammatory factors. Spinal cord injury (SCI) is damage to the spinal cord caused by the trauma or disease that results in compromised or loss of body function. Subsequent to SCI in humans, many individuals have residual motor and sensory deficits that impair functional performance and quality of life. The available treatments for SCI are rehabilitation therapy, activity-based therapies, and pharmacological treatment using antioxidants and their agonists. Among pharmacological treatments, the most efficient and commonly used antioxidant for experimental SCI treatment is melatonin, an indolamine secreted by pineal gland at night. Melatonins receptor independent free radical scavenging action and its broad-spectrum antioxidant activity makes it an ideal antioxidant to protect tissue from oxidative stress-induced secondary damage after SCI. Owing to the limitations of an activity-based therapy and antioxidant treatment singly on the functional recovery and oxidative stress-induced secondary damages after SCI, a melatonin plus exercise treatment may be a more effective therapy for SCI. As suggested herein, supplementation with melatonin in conjunction with exercise not only would improve the functional recovery by enhancing the beneficial effects of exercise but would reduce the secondary tissue damage simultaneously. Melatonin may protect against exercise-induced fatigue and impairments. In this review, based on the documented evidence regarding the beneficial effects of melatonin, activity based therapy and the combination of both on functional recovery, as well as reduction of secondary damage caused by oxidative stress after SCI, we suggest the melatonin combined with exercise would be a novel neuro-rehabilitative strategy for the faster recovery after SCI [12].
Samuel Davi, et al: CNS injury-induced hemorrhage and tissue damage leads to excess iron, which can cause secondary degeneration. The mechanisms that handle this excess iron are not fully understood. We report that spinal cord contusion injury (SCI) in mice induces an “iron homeostatic response” that partially limits iron-catalyzed oxidative damage. We show that ceruloplasmin (Cp), a ferroxidase that oxidizes toxic ferrous iron, is important for this process. SCI in Cp-deficient mice demonstrates that Cp detoxifies and mobilizes iron and reduces secondary tissue degeneration and functional loss. Our results provide new insights into how astrocytes and macrophages handle iron after SCI. Importantly, we show that iron chelator treatment has a delayed effect in improving locomotor recovery between 3 and 6 weeks after SCI. These data reveal important aspects of the molecular control of CNS iron homeostasis after SCI and suggest that iron chelator therapy may improve functional recovery after CNS trauma and hemorrhagic stroke [13].
Rajiv R Ratan, et al: Stroke is the leading cause of disability in the United States, and yet no definitive interventions can drive the nervous system beyond its measurable but often limited spontaneous recovery. Treatment to limit injury progression and enhance repair after stroke or other types of central nervous system injury is complicated by the heterogeneous nature of cell death and wound healing mechanisms and the multiple barriers to functional recovery. The heterogeneity of injury and repair mechanisms requires interventions that are broad and multi-modal, but also intrinsically safe. We describe a process to identify such interventions by screening multiple individual targets in the historically separate realms of neuroprotection, repair, and regeneration against a library of FDA-approved compounds with known safety. We have identified nearly 10 compounds that are able to activate simultaneously protective and reparative genes. These compounds have a theoretical therapeutic window that spans from evolving injury (minutes to hours) to stable injury (days to months to years). It is our hypothesis that these compounds will be most efficacious when paired with training. The notion is that drugs will alter the propensity of the nervous system toward recovery, whereas specific training will engage the needed instructive cues to achieve this goal. Indeed, robotic training can provide a level of motor learning that seems to enhance the salutary effects of training. In a system that depends heavily on cells that do not easily replenish themselves, cellular therapies could also ultimately be an important part of the cocktail. We conclude that combinations of interventions will be needed to surmount [14].
Thomas Goetz, et al: Paraplegias of traumatic origin may be classified as primary or secondary. Secondary traumatic paraplegia (STP) is believed to result from an auto destructive process. Different authors have published results supporting or contradicting the therapeutic effects of durotomy alone or associated with exposed spinal cord and perfusion with a saline solution at normal or cold temperatures. It appears that although decompression and open dialysis might be beneficial, the surgical trauma over the injured region is detrimental. A method of local epidural spinal cord cooling has been developed and successfully used to treat STP. With this method, no surgical injury or damage is imposed on the dura, cerebro-spinal fluid (CSF), or spinal cord. Several of the beneficial effects attributed to hypothermia in the traumatized area are evident, including reduction of metabolic demands, edema, swelling, vasospasm, and blood pressure. Aware of the benefits that dialysis may have in STP, as well as of the encouraging results obtainable with local epidural spinal cord cooling, we hypothesized that this method of hypothermia may in some way trigger CSF dialysis. Based on this hypothesis, a model was developed approximating the behavior of the CSF in the situation where a cold source is applied to the dura. Using dimensionless analysis techniques, we predict that CSF under the cooled region of the dura undergoes convective motion, even in adverse situations where the spinal cord has swollen. Under steady-state conditions, the moving fluid forms several Bénard cells directly under the cold source. The size of these Bénard cells was estimated. The range of probe temperatures at which convective flow is generated was considered, as well as the relative benefits of hypothermia versus flow. Results of more rigorous analysis are discussed [15].
Patrick Freund, et al: The Neurobiology Underlying Plasticity in the Injured Spinal Cord. Despite the detrimental impact of trauma, the axonal architecture of the spinal cord undergoes a cascade of dynamic (short term and long term) regenerative mechanisms that have been linked to spontaneous functional recovery. An essential anatomical feature—underlying this recovery from SCI—is synaptic plasticity of preexisting connections rewiring of injured fiber tracts transient down-regulation of the Nogoreceptor-1 signaling cascade and the formation of new “detour circuits”. Experimental evidence suggests that axonal remodeling and plasticity occurs not only within the spinal cord below and above the lesion but also within the brain both subcortically and cortically. At the cervical level, collateral cortico-spinal sprouts emerge about 10 days after injury and may connect to interneurons within 3 months. The early formation of corticospinal sprouts is in line with the acute effects on brain activation changes in complete thoracic “lesioned” rodents, where cortico-spinal hindlimb fibers are rewired—changing into forelimb fibers. Here the cortical representation of the unimpaired, overused forepaw in the ipsilesional cortex was enhanced at 3 months. The formation of detour circuits—which encompass the lesion in spared tissue—could reconnect to locomotor circuits, thus enabling afferent input to be processed and conveyed to the cortex. Indeed, clinical findings suggest that these detour formations could be the substrate for improved spinal reflexes, even below the injury in incomplete SCI patients. Promoting regenerative sprouting, to restore function, has been a major goal over decades. Recently, several interventions have entered clinical trials that either aim to protect neurons or to foster regeneration Treatment induced evidence for functionally meaningful connectivity is sparse but has been established for regenerating sensory axons that reconnect with dorsal horn neurons and for corticospinal axons regenerating below the level of lesion. Moreover, no adverse effects were observed following the anti-Nogo-A antibody treatment in nonhuman primates and in a clinical phase I trial in SCI patients [16].
Geoffrey Raisman, et al: Many tissues of the body are capable of self-repair. Skin, bone, gut, and others can recover from injury. But despite all efforts of the patient and the medical profession, the tissues of the brain and spinal cord—which together constitute the central nervous system—show major and irreversible loss of function if they are damaged.In approaching this matter we encounter 2striking and prevalent ideas. The first is that, although the embryonic central nervous system is able to form new connections, it loses the ability at some time after birth. The second is that the adult central nervous system is full of inhibitory molecules which prevent regeneration of damaged nerve fibres. Together these truly represent a pessimistic view. What is so special about cells from the olfactory system? For many years it was thought that we are born with a full complement of nerve cells, and that the only change we can expect during life is to lose them. But the advent of labels to detect cells undergoing division showed that new neurons are continually added to the olfactory system of the adult brain—an initially derided finding that took 20 years to become accepted. The same methodology also revealed that the neurosensory cells of the olfactory mucosa have a life of about thirty days, and are continually replaced by the progeny of mucosal stem cells generated throughout adult life.The continuous death and replacement of olfactory neurons means that the olfactory nerves are likewise in a state of continuous replacement. And this raises the question of how these newly formed nerves are able to enter the brain. In 1985 I described a unique arrangement of specialized olfactory ensheathing glial cells that accompany the olfactory nerve fibres all the way to their entry into the brain. The subsequent development of a tissue culture method for obtaining olfactory ensheathing cells from adult olfactory tissue samples led to a mixture of 2 main types of cells, some Schwann-like, others fibroblast-like, which could be transplanted into experimental lesions of the spinal cord. In our own studies we transplanted this mixture of cultured olfactory ensheathing cells into complete unilateral lesions of the upper cervical corticospinal tract in adult rats. We found that the grafted cells encourage the growth of the cut nerve fibres, and suppress the excessive neuromatous branching found in untreated lesions. The grafted cells take up an elongated shape and form a tightly aligned bridge between the ends of the cut fibre tract. The regenerating nerve fibres enter the graft and follow this new aligned bridge pathway. Within the bridge the nerve fibres are intimately ensheathed by the Schwann-like cells, and 260 enclosed in an outer, perineurial-like, sheath of fibroblasts. But, most important, once they reach the end of the graft they re-enter the host spinal cord and continue along the distal part of the corticospinal tract to form terminal arborization in their normal target areas. During their course through the transplant the fibres are myelinated by peripheral myelin formed by the Schwann-like cells, and when they re-enter the spinal cord they are myelinated by host OLIGO-DENDROCYTES. The effect is to put a patch over the lesion, restoring the integrity of the original pathway. This regeneration can be achieved by transplanting at some time after the original injury, and leads to the functional recovery of a learned task [17].
Camandola S, et al: Brain cells normally respond adaptively to bioenergetic challenges resulting from ongoing activity in neuronal circuits, and from environmental energetic stressors such as food deprivation and physical exertion. At the cellular level, such adaptive responses include the “strengthening” of existing synapses, the formation of new synapses, and the production of new neurons from stem cells. At the molecular level, bioenergetic challenges result in the activation of transcription factors that induce the expression of proteins that bolster the resistance of neurons to the kinds of metabolic, oxidative, excitotoxic, and proteotoxic stresses involved in the pathogenesis of brain disorders including stroke, and Alzheimer’s and Parkinson’s diseases. Emerging findings suggest that lifestyles that include intermittent bioenergetic challenges, most notably exercise and dietary energy restriction, can increase the likelihood that the brain will function optimally and in the absence of disease throughout life. Here, we provide an overview of cellular and molecular mechanisms that regulate brain energy metabolism, how such mechanisms are altered during aging and in neuro-degenerative disorders, and the potential applications to brain health and disease of interventions that engage pathways involved in neuronal adaptations to metabolic stress [18].
Farron L McInteem, et al: Amyloidb (Ab) is the major constituent of the brain deposits found in parenchymal plaque and cerebral blood vessels of patients with AD. Several lines of investigation support the notion that synaptic pathology, one of the strongest correlates to cognitive impairment, is related to the progressive accumulation of neurotoxic Ab oligomers. Since the process of oligomerization/fibrillization is concentration-dependent,it is highly reliant on the homeostatic mechanisms that regulate the steady state levels of Ab influencing the delicate balance between rate of synthesis, dynamics of aggregation, and clearance kinetics. Emerging new data suggest that reduced Ab clearance, particularly in the aging brain, plays a critical role in the process of amyloid formation and AD pathogenesis. Using well-defined monomeric and low molecular mass oligomeric Ab1-40 species stereotaxically injected into the brain of C57BL/6wild-type mice in combination with biochemical and mass spectrometric analyses in CSF, our data clearly demonstrate that Ab physiologic removal is extremely fast and involves local proteolytic degradation leading to the generation of heterogeneous C-terminally cleaved proteolytic products, while providing clear indication of the detrimental role of oligomerization for brain Ab efflux. Immunofluorescence con focal microscopy studies provide insight into the cellular pathways involved in the brain removal and cellular uptake ofAb.The findings indicate that clearance from brain interstitial fluid follows local and systemic paths and that in addition to the blood-brain barrier, local enzymatic degradation and the bulk flow transport through the choroid plexus into the CSF play significant roles. Our studies highlight the diverse factors influencing brain clearance and the participation of various routes of elimination opening up new research opportunities for the understanding of altered mechanisms triggering AD pathology and for the potential design of combined therapeutic strategies. The most frequent form of amyloidosis in humans is related to the deposition of amyloid-b (Ab) in AD, with cumulative biochemical, genetic, and in vivo data strongly suggesting a central role for this molecule in the pathogenesis of the disorder. Ab is the major constituent of the tissue deposits found in parenchymal plaques and cerebral blood vessels of patients with A Dand with Down’s syndrome, the latter exhibiting a Trisomyinchromosome 21 which codes for the Amyloid Precursor Protein (APP) and lead to AD pathology by middle Age Indeed, Ab I s an internal processing product of this transmembrane APP. Precursor molecule generated through proteolytic cleavage by the b-and g-secretases. Although it is unclear what primarily triggers and drives the progression of AD, histo pathologic, genetic, biochemical, and physicochemical studies, together with information obtained from transgenic animal models, strongly support the notion that abnormal aggregation/fibrillization and subsequent Ab tissue accumulation are keyp layers in the disease pathogenesis.
Although the abundance of mature amyloid plaques correlates poorly with AD severity. Current Data indicate that the transition from soluble monomeric species normally found in circulation to oligomeric, protofibrillar, and end-pointfibrillar assemblies contribute significantly to disease pathogenesis. Intermediate oligomeric and protofibrillar forms, in particular seem to display the most potent effects in neuronal cells, inducing synaptic disruption and neurotoxicity. Numerous studies Have shown that the sesoluble oligomeric forms ofAb—which Have been identified in vivo and isolated from brain, plasma and CSF—are capable of affecting synaptic function by various mechanisms, impairing glutamatergic synaptic transmission strength and plasticity, altering synaptic structure, reducing efficacy of synapses and causing synaptic loss. The process of oligomerization/fibrillization is concentration- dependent and therefore it is highly reliant on the homeostatic mechanisms that regulate the steady state levels of Ab modulating the delicate balance between rate of synthesis, dynamics of aggregation and rate of brain efflux. For the majority of AD cases, which are of late-onset and of sporadic origin the cause of this imbalance is unclear and remains a subject of active investigation. While to date no evidence supports an increase in the overall production in sporadic cases, current research suggests that an impaired clearance in late onset AD plays a critical role in the process of amyoid formation and the pathogensis of the disease. Many pathways are currently being investigated among them Peri-vascular drainage receptor-mediated celluptake blood Brain barrier (BBB) transport and local proteolytic degradation, All undoubtedly contributors to brain Ab clearance in Conjunction with the bulk flow of ISF into the CSF through the Choroid plexus epithelium which Remarkably shares many of The receptors involved inBBB clearance as well as the recently described paths for CSF recycling through the ISF. Notably, in Spite of the relevance of Ab oligomerization for the disease pathogenesis, the vast majority of the reported Ab clearance data have been generated with monomeric Ab species or with peptides with poorly characterized aggregation state. The Present work was designed to address this gap in knowledge and provide quantitative evaluation of the differential brain Removal efficiency of pathogenic oligomeric Ab assemblies while providing information into the relevance of the in situ catabolic break-down of the peptide. Based on data from intra-cerebral stereotaxic injections of well-defined monomeric and low molecular mass oligomeric Ab assemblies inC57BL/6 wild-type mice in combination with biochemical and mass spectrometry analyses in CSF, the current work highlights the fast physiologic degradation and brain elimination of the peptide and provides a clear indication of the Detrimental role of oligomerization for brain Ab efflux. Immuno-fluorescence con focal microscopy studies bring insight into the cellular pathways involved in the brain clearance and cellular uptake of Ab [19].
Walker S Jackson, et al: The mechanisms underlying the selective targeting of specific brain regions by different neuro-degenerative diseases is one of the most intriguing mysteries in medicine. It is known that Alzheimer’s disease primarily affects parts of the brain that play a role in memory, whereas Parkinson’s disease predominantly affects parts of the brain that are involved in body movement. The reasons that other brain regions remain unaffected in these diseases are unknown. A better understanding of the phenomenon of selective vulnerability is required for the development of targeted therapeutic approaches that specifically protect affected neurons, thereby altering the disease course and preventing its progression. Prion diseases are a fascinating group of neuro-degenerative diseases because they exhibit a wide phenotypic spectrum caused by different sequence perturbations in a single protein. The possible ways that mutations affecting this protein can cause several distinct neuro-degenerative diseases are explored in this Review to highlight the complexity underlying selective vulnerability. The premise of this article is that selective vulnerability is determined by the interaction of specific protein conformers and region-specific microenvironments harboring unique combinations of subcellular components such as metals, chaperones and protein translation machinery. Given the abundance of potential contributory factors in the neuro-degenerative process, a better understanding of how these factors interact will provide invaluable insight into disease mechanisms to guide therapeutic discovery [20].
Martin Marsala, et al: We examined the effect of reversible spinal cord ischemia on extracellular amino acid release, SCBF, and early histo-pathological changes in rat. Glutamate was significantly elevated 10 min after aortic occlusion, corresponding with the state of incomplete spinal cord ischemia as measured by the laser Doppler technique. Probably as a result of excitotoxic- induced neuronal damage, the taurine concentration was significantly elevated during the entire reperfusion period. Histo-pathological analysis revealed selective neuronal damage affecting small and medium-sized neurons, possibly GABA ergic interneurons, localized predominantly in laminae III-VI, corresponding with the signs of irreversible neuronal damage on an ultra-structural level. These results suggest that the measurement of extracellular levels of amino acids may provide insights into mechanisms and into correlation with function. Thus, in subsequent experiments, pharmacological and physiological manipulations that alter neuronal transmitter release can be compared with the effects of these manipulations on time-dependent changes in spinal histopathology and function. Amino acid changes during and after ischemia Glutamate is believed to be the primary EAA transmitter of the spinal cord; it has been localized in a subpopulation of primary afferent fibers and in certain descending tracts. The significant elevation in glutamate concentration during ischemia is in agreement with a number of experimental studies in which irreversible postischemic neuronal damage has been postulated to be linked with NMDA receptor overstimulation. Recent in vitro experiments have shown that glutamate-mediated neuronal damage can be divided into three distinct phases: (a) an initial phase when the neuron is transiently exposed to an increased concentration of glutamate and the intra-cellular Ca2+ increases to micromolar levels, followed by (b) a latent phase during which the intra-cellular Ca2+ recovers to basal levels and (c) a terminal phase characterized by a gradual rise in Ca2+ in the intra-cellular space, which reaches a prolonged plateau. The last phase correlates significantly with cell death. In the present study glutamate increased significantly only after 10 min of occlusion. This finding, in accordance with SCBF data, suggests that aortic occlusion in the rat leads to incomplete spinal cord ischemia and that a relatively prolonged occlusion time under these conditions is necessary to evoke anoxic neuronal depolarization followed by significant glutamate release. This release, although significant (150-200% of the preischemic control), is of a smaller magnitude than that reported in the hippocampal CA l region, where glutamate increases 15- to 18-fold during global cerebral ischemia. Despite the fact that spinal cord glutamate exhibited only modest increases during 20 min of ischemia (1.5- to 2-fold), the histopathology in these animals sacrificed after only 2 h of reperfusion showed clear signs of irreversible neuronal damage.
Hippocampal CA l neurons, in contrast, release massive quantities of glutamate during and immediately after an equivalent duration of global ischemia, yet irreversible neuronal changes as indicated by heavy neuronal argyrophilia, neuronal eosinophilia, or complete loss of neurons occurs only after 2-3 days of reperfusion. Thus, it appears likely that in the spinal cord, additional factors may potentiate the development of neuronal death during the early reperfusion period. During the initial 30-60 min post-ischemia, glutamate usually returned to close to pre-ischemic concentrations, presumably due to a reuptake system. This decrease in extracellular glutamate concentration does not exclude the possibility that NMDA receptors remain activated. This “latent” activation may in turn evoke secondary Ca2+ influx. Surprisingly, conditions of complete spinal cord ischemia as evoked by cardiac arrest evoked only a 350-400% increase in the pre-arrest glutamate concentration. These data show that the total amount of glutamate released into the extracellular space under conditions of complete spinal cord ischemia is substantially lower than those seen in supra-spinal structures under comparable conditions. Importantly, in recent studies, it has been shown that the spinal delivery of exogenous NMDA enhances spinal glutamate release. This provides a mechanism whereby a positive feedback circuit might induce and maintain increased NMDA and non-NMDA receptor activation. Glycine is a potent spinal cord inhibitory transmitter localized mostly in small dorsal horn neurons the modest increases in glycine that we observed during reperfusion are in agreement with a number of studies in which transient cerebral ischemia evoked significant elevations of glycine during the post-ischemic recirculation period [21].
L Sakkaa, et al: The cerebro-spinal fluid (CSF) is contained in the brain ventricles and the cranial and spinal subarachnoid spaces. The mean CSF volume is 150 ml, with 25 ml in the ventricles and 125 ml in subarachnoid spaces. CSF is predominantly, but not exclusively, secreted by the choroid plexuses. Brain interstitial fluid, ependyma and capillaries may also play a poorly defined role in CSF secretion. CSF circulation from sites of secretion to sites of absorption largely depends on the arterial pulse wave. Additional factors such as respiratory waves, the subject’s posture, jugular venous pressure and physical effort also modulate CSF flow dynamics and pressure. Cranial and spinal arachnoid villi have been considered for a long time to be the predominant sites of CSF absorption into the venous outflow system. Experimental data suggest that cranial and spinal nerve sheaths, the cribriform plate and the adventitia of cerebral arteries constitute substantial pathways of CSF drainage into the lymphatic outflow system. CSF is renewed about four times every 24 hours. Reduction of the CSF turnover rate during ageing leads to accumulation of catabolites in the brain and CSF that are also observed in certain neuro-degenerative diseases. The CSF space is a dynamic pressure system. CSF pressure determines intracranial pressure with physiological values ranging between 3 and 4mmHg before the age of one year, and between 10 and 15mmHg in adults. Apart from its function of hydromechanical protection of the central nervous system, CSF also plays a prominent role in brain development and regulation of brain interstitial fluid homeostasis, which influences neuronal functioning. Cerebro-spinal fluid homeostasis CSF exerts a well-known function: hydromechanical protection of the neuraxis. CSF plays an essential role in homeostasis of cerebral interstitial fluid and the neuronal environment by regulation of the electrolyte balance, circulation of active molecules,and elimination of catabolites. CSF transports the choroidal, plexus secretion products to their sites of action. This mode of distribution by CSF circulation modulates the activity of certain regions of the brain by impregnation, while synaptic transmission produces more rapid changes of activities. The wastes of brain metabolism, peroxidation products and glycosylated proteins, accumulate with age-relateddecreased CSF turnover [22].
Jenna M. Tarasoff, et al: Accumulation of toxic protein aggregates—amyloid-β (Aβ) plaques and hyper-phosphorylated tau tangles—is the pathological hallmark of AD. Aβ accumulation has been hypothesized to result from an imbalance between Aβ production and clearance; indeed, Aβ clearance seems to be impaired in both early and late forms of AD. To develop efficient strategies to slow down or halt AD, it is critical to understand how Aβ is cleared from the brain. Extracellular Aβ deposits can be removed from the brain by various clearance systems, most importantly, transport across the blood–brain barrier. Past Findings suggest that astroglial-mediated interstitial fluid (ISF) bulk flow, known as the glymphatic system, might contribute to a larger portion of extracellular Aβ (eAβ) clearance than previously thought. The meningeal lymphatic vessels, discovered in 2015, might provide another clearance route. Because these clearance systems act together to drive eAβ from the brain, any alteration to their function could contribute to AD. An understanding of Aβ clearance might provide strategies to reduce excess Aβ deposits and delay, or prevent, disease onset. The removal of soluble waste from the brain occurs via various overlapping clearance systems, which can be classified according to the compartment from which the waste is directly cleared, and the compartment into which the waste is directly cleared. Protein waste can be cleared from the intra-cellular compartment, or from the extracellular compartment, which comprises the ISF that surrounds neurons and the CSF that surrounds the brain. These proteins can then be removed by enzymes or cellular uptake, exported into the blood or lymph, or re-circulated in the CSF. The relative contributions of each of the various clearance systems are currently unknown; the prevailing view is that the BBB clearance predominates, though recent studies involving peri-vascular CSF circulation challenge this view. Degradation clearance is the enzymatic breakdown of proteins in the brain, and entails both extra-cellular and intra-cellular degradation. Extracellular degradation of ISF proteins mainly consists of degradation by proteases expressed and secreted by cells such as astrocytes. ISF proteins can also be taken up from the extracellular space to be degraded intra-cellularly in neurons or glia, including phagocytic microglia and astrocytes. Intra-cellular degradation of proteins occurs via the ubiquitin– proteasome pathway, the autophagy–lysosome pathway, and the endosome–lysosome pathway. Blood–brain barrier clearance interstitial proteins can be cleared into the blood directly at the BBB through specialized transport systems located in the brain endothelium. The BBB endothelial cells are connected by tight junctions and have 2 functionally distinct sides: the luminal side facing the blood circulation, and the abluminal side facing the brain parenchyma.In addition to the BBB, the brain is also protected by the so-called ‘glial barrier’ (also known as the glia limitans) that surrounds the BBB and consists of astroglial end feet processes that cover the majority of the parenchymal vasculature, with the remaining area consisting of intercellular astrocytic end feet clefts forming gap junctions.
The BBB and glial barrier are part of the neuro-vascular unit, including cerebral micro-vascular endothelium, basement membrane, contractile pericytes (which share the capillary basement membrane with the endothelium), smooth muscle cells (which invest the endothelium of pre-capillary arterioles), astroglia, and neurons. Transport at the neurovascular unit across the glial barrier and BBB depends on the solubility, molecular weight and diameter of the protein. The relatively large size of the intercellular clefts (20 nm) implies that the glial barrier is permeable to nearly all proteins. Given the size of AD-related proteins, monomeric Aβ1–40, Aβ1–42 and tau, should be able to pass freely through astrocytic end feet clefts at the glial barrier. Endothelial tight junctions at the BBB prevent free passage of Aβ and tau into the blood, so they must instead be transported across the endothelium by specialized transporters, which, as descried below, have been identified for Aβ, but not for tau. Interstitial fluid bulk-flow clearance ISF proteins can also be cleared directly into the CSF via ISF bulk flow that enters the CSF sink or the peri-vascular space. Cerebro-spinal fluid sink clearance—in parts of the body other than the CNS, lymphatic vessels run in parallel with the circulatory system to clear waste from the ISF in the form of lymph. Lymphatic vessels have recently been described in the meninges surrounding the mouse brain, but the brain parenchyma itself is devoid of such vessels, leading to the long-held assumption that CSF serves as a ‘lymph equivalent’ to clear waste from the CNS. Apart from transport across the endothelium, the removal of ISF from the brain parenchyma was traditionally believed to occur by diffusion or ISF bulk flow into the CSF sink, which comprises the ventricles and subarachnoid space. Given that diffusion is dependent on molecular size, diffusion into the CSF sink has been proposed to be too slow for the highly metabolic and large human brain. ISF bulk flow, which is independent of molecular size, has been proposed as the predominant pathway for movement of large molecules into the CSF sink. ISF bulk flow was initially thought to course through the brain in a diffuse manner, but later evidence suggests the existence of definite pathways. Peri-vascular clearance—Support for an anatomically specific bulk-flow system came from a study of peri-vascular circulation. Following infusion of horseradish peroxidase into the lateral ventricles or subarachnoid space of anaesthetized cats and dogs, CSF within the subarachnoid space flowed freely through the Virchow–Robin space—a histologically defined space where the subarachnoid space meets the peri-vascular space. From the Virchow–Robin space, CSF travelled into the peri-arterial spaces that surround penetrating arteries, moving along specific pathways in the same direction as blood flow. CSF was also shown to move from the peri-vascular space into the interstitial ISF. This peri-vascular circulation hypothesis challenged the traditional model of one-way flow of ISF into the CSF sink. Mouse model and human studies involving fluorescent soluble tracers and confocal microscopy have demonstrated that following intra-cerebral injection, ISF solutes diffuse and enter peri-vascular drainage pathways along the basement membrane of capillary and arterial walls separating smooth muscle cells, then move towards the lepto-meningeal arteries at the surface of the brain and, ultimately, to cervical lymph nodes . This pathway was named the peri-vascular drainage pathway, and was deemed to be the lymphatic drainage of the brain. The peri-vascular circulation hypothesis was recently confirmed and expanded on by a study in mice. Following tracer injection into the CSF at the cisterna magna, 2-photon microscopy was used to visualize in real time the flux of CSF in living mice through a closed cranial window. As already hypothesized decades ago, CSF was found to act like lymph: it flushed out interstitial substances in a process facilitated by glial cells, prompting the authors to name it the glymphatic system.
This evidence corroborated prior findings that CSF, driven by arterial pulsation, flows into the periarterial space, following the course of the arterial vascular smooth muscle basement membrane to reach the basal lamina of the brain capillary bed, and entering the interstitium at all levels of this peri-vascular route.The work also confirmed that ISF moves by bulk flow.the study extended these findings to include a role for astroglial AQP4 channels. These channels were found to mediate CSF transport from the peri-arterial space across the pial–glial membrane into the interstitium, where it mixes with ISF. The pia has been shown to be relatively permeable: tracers injected into the subarachnoid space rapidly enter the peri-vascular space and brain parenchyma. CSF–ISF movement from the interstitium into the peri-venous space of deep draining veins runs ventro-medially towards ventricular and deep white matter structures. Hydrostatic pressure of peri-arterial bulk flow has been speculated to drive CSF water through the AQP4 channels, which is followed by astrocytic passage of molecules both through clefts and across astrocytes to maintain osmotic balance, although the mechanism has not been fully elucidated. Any remaining CSF components course along the capillary basal lamina.It is unclear whether the peri-vascular drainage pathway and the glymphatic pathway are in fact distinct, or simply reflect transport along the same pathway captured under differing physiological or experimental conditions. In other study using 2-photon imaging, fluorescent tracers were injected directly into the mouse brain via an open skull. Peri-arterial tracer accumulation was observed, although the direction of the flow was not discerned. Opening of the skull is suggested to be a confounding factor in many experiments involving circulation of CSF, because skull removal can lead to inflammation and mechanical injuries to the cortical surface, or disturb local blood perfusion, BBB permeability and brain homeostasis. The peri-vascular drainage and glymphatic pathways are not mutually exclusive: both could be active depending on the conditions, and the pathway in use could even be different between vessels or within the same vessel at different times . Cerebro-spinal fluid absorption clearance following clearance from the ISF into the CSF, proteins must be cleared from the brain. Circulating CSF can be absorbed directly into the circulatory or lymphatic systems. Circulatory clearance—Although other CSF production sites have also been suggested, the majority of CSF seems to be produced at the BCSFB by the choroid plexus, a vascular unit of capillaries comprised of fenestrated endothelium and covered by choroid plexus epithelium (modified ependymal cells with tight junctions), located in the ventricles. The BCSFB serves not only as a CSF production site, but also as a ventricular CSF solute clearance site. According to the traditional point of view, following CSF production by the choroid plexus, CSF circulates within the subarachnoid space, from where it is primarily cleared from the brain at arachnoid villi-one-way valve structures leading to the dural venous sinuses. Lymphatic clearance.
As described above, circulating CSF within the peri-vascular space can be cleared from the brain to cervical lymph nodes. Another clearance route for circulating CSF to cervical lymph nodes is along peri-neural spaces, extensions of the subarachnoid space surrounding nerves. the recent development of a method for mounting of whole meninges, such that mouse meninges can be examined intact on a single slide, led to the discovery of meningeal lymphatic vessels, which might provide another clearance route for circulating CSF proteins.These meningeal lymphatic vessels might also provide a more conventional path for immune cells to exit the CNS, and dysfunction of these vessels might have important implications for neurological disorders associated with altered immune responses. Degradation clearance Intra-cellular Aβ (iAβ) can be degraded by proteasomes via the ubiquitin–proteasome pathway in neurons, lysosomal cathepsin enzymes, proteases (such as insulin-degrading enzyme, a thiol metallo-endo-peptidase that degrades monomeric Aβ) and insulin. Extracellular Aβ can also be degraded by proteases, such as neprilysin (a membrane-anchored zinc metallo-endopeptidase that degrades the Aβ monomers Aβ1–40 and Aβ1–42, and Aβ oligomers), matrix metallo-proteinases, glutamate carboxy-peptidase II, endothelin-converting enzyme, tissue plasminogen activator, plasmin, angiotensin-converting enzyme, and insulin-degrading enzyme. In addition, eAβ can be degraded following glial phagocytosis. Specifically, ISF Aβ can be taken up by microglia and astrocytes, whereas peri-vascular Aβ can be degraded by vascular smooth muscle cells, peri-vascular macrophages, and astrocytes .Degradation clearance of Aβ is affected by four main factors: enzyme expression and activity, ligand affinity and competition, activation of cellular uptake, and initiation of intra-cellular degradation pathways all of which become impaired with ageing and in AD. Expression of neprilysin is decreased in AD, especially in regions with high Aβ loads such as the hippocampus and temporal gyrus. overall matrix metallo-proteinase 2 expression is increased in AD,58 its activity is reduced in astrocytes that surround Aβ plaques.both Aβ and insulin are ligands that compete for degradation by insulin-degrading enzyme; hyper-insulinaemia can reduce the clearance of Aβ, which might partly explain the link between type 2 diabetes mellitus and AD. Plaques activate the immune effectors of the CNS—microglia and astrocytes—inducing both phagocytosis of Aβ, which facilitates clearance from the extracellular space, and production of neurotoxic inflammatory cytokines. Aβ that has undergone cellular uptake can then be degraded, for example via the autophagy–lysosome pathway or be released back into the extracellular space,as found in the brains of patients with AD. In AD, Aβ degradation via the endosome–lysosome pathway is increased relative to lysosomal degradation: endocytic activity is elevated, resulting in accumulation of autophagic vacuoles, presence of lysosomal cathepsin enzymes in Aβ plaques, and abnormally enlarged endosomes containing Aβ, leading to generalized proteasome dysfunction. Blood-brain barrier clearance Mechanisms of amyloid-β influx and efflux—Aβ is transported from the interstitial space across the BBB and into blood, and vice versa. Specifically, local soluble Aβ is transferred from the interstitium to the brain by LDL receptor (LDLR) family members such as LRP1, and ATP-binding cassette transporters (ABC transporters). Evidence suggests that LRP1 is the main transporter for Aβ efflux at the BBB.
The main ABC transporter responsible for Aβ efflux is ABCB1 (also known as P-glycoprotein 1 or MDR1), which directly exports Aβ into the circulation. ABCA1, which is located on the abluminal side of the brain endothelium,does not directly bind and extrude Aβ, but mediates Aβ clearance in an ApoE-dependent manner. The precise mechanism by which abluminal ABCA1 mediates Aβ clearance is unknown, although this transporter has been proposed to induce ApoE lipidation, which facilitates ApoE-Aβ interaction in the peri-vascular space, making Aβ more accessible to transport by LRP1 or ABCB1. Clearance of Aβ through the BBB is also mediated by α2-macroglobulin (α2M), 14 and LDLR-related protein 2 (LRP2, also known as megalin) when LRP2 forms a complex with clusterin (also known as ApoJ).insulin-degrading enzyme has been proposed to have a role in Aβ clearance through the BBB, which might explain why BBB clearance is sensitive to insulin.Free Aβ can be transported from the circulation into the interstitium via RAGE (advanced glycosylation end product-specific receptor). Soluble transporters (also known as sequestering agents)—such as the soluble form of RAGE (sRAGE), anti-Aβ IgG, serum amyloid P component (SAP), and the soluble form of LRP (sLRP), which binds 70–90% of plasma Aβ—bind to soluble Aβ and inhibit its binding to RAGE, thereby preventing Aβ from entering the interstitium.Factors impairing amyloid-β clearance in AD—Clearance of Aβ through the BBB is affected by transporter expression and activity, ligand affinity and competition, and vascular integrity . In AD, these factors are impaired in a number of ways. Expression of the blood efflux transporters LRP1 and ABCB1 is decreased, whereas expression of the blood influx transporter RAGE is upregulated. Oxidative changes in AD are linked to changes in sLRP that reduce its affinity for Aβ, potentially facilitating Aβ influx into the interstitium by RAGE. Inflammation, a common feature of AD, can affect ligand affinity by making the pH more acidic, which promotes hyper-phosphorylation of tau and induces conformational changes in Aβ that hinder its clearance. ApoE is a cholesterol transporter that competes with Aβ for efflux by LRP1 from the interstitium into the circulation;competition for shared receptors is the primary mechanism by which ApoE mediates Aβ clearance. The strongest genetic risk factor for AD is APOE*ε4152 (APOE*ε4>APOE*ε3>APOE*ε2151), which codes for an ApoE isoform that is less efficient at mediating Aβ clearance than are the other ApoE isoforms.Third, ApoE4 is also associated with lower antioxidant activity than other ApoE isoforms, and it mediates BBB breakdown through a proinflammatory pathway involving cyclophilin A in pericytes. These findings are in line with evidence suggesting that increased oxidative stress and loss of vascular integrity contribute to ageing and AD, as demonstrated by accelerated breakdown of the BBB and the neurovascular unit. Interstitial fluid bulk-flow clearance ISF bulk-flow clearance removes ISF—which contains eAβ—from the interstitium via ISF bulk flow into the CSF sink and peri-vascular space. We will discuss peri-vascular clearance of Aβ specifically via the peri-vascular drainage and glymphatic pathways.Peri-vascular drainage—Aβ is cleared along peri-vascular drainage pathways. In both AD and CAA44 (commonly associated with AD84), peri-vascular drainage of Aβ is impaired. Known factors affecting peri-vascular drainage of Aβ include APOE*ε4, deposition of immune complexes, arterial age, and—possibly—arterial pulsation. The presence of ApoE4 is associated with reduced peri-vascular drainage of Aβ, which in turn is linked to deposition of immune- complexes. Peri-vascular drainage of Aβ fails as arteries age; this failure is associated not only with loss of homeostasis and elevated levels of soluble Aβ in the brain, but also with accumulation of Aβ in arterial walls (as seen in CAA), which increases the risk of intra-cerebral lobar haemorrhages. One of the main complications following immunization against Aβ is the solubilization of Aβ from plaques and entrapment in peri-vascular drainage pathways, which worsens CAA. It is possible that arterial pulsation drives peri-vascular drainage of ISF solutes and that morphological changes associated with age-related arterio-sclerosis result in failure of peri-vascular drainage. Of note, a high-fat prenatal maternal diet has recently been reported to result in a failure of Aβ clearance along cerebrovascular basement membranes.
This failure was exacerbated if the high-fat diet had been lifelong, suggesting a role for epigenetic changes and diet in AD patho-genesis. Glymphatic clearance-recent mouse studies suggest that the AQP4-dependent glymphatic pathway is an important clearance system for driving the removal of soluble Aβ from the interstitium. In mice, Aβ is cleared along peri-vascular pathways, and Aβ clearance was reduced by 55–65% in Aqp4 knockout mice compared with wild-type mice. glymphatic clearance was reduced by 40% in aged relative to young mice, suggesting that the glymphatic pathway is impaired with age, which, as mentioned above, is the primary risk factor for LOAD.Potential factors affecting glymphatic ISF bulk flow include molecular size, arterial pulsation, AQP4 expression and localization, and sleep . Following subarachnoid injection, larger tracer molecules are slower to enter the parenchyma than are smaller tracers, and soluble peri-vascular Aβ can cross the 20 nm astrocytic end feet clefts. Arterial pulsation is critical for peri-vascular circulation and transport of CSF into the interstitium. Recirculating Aβ-rich CSF within the peri-arterial space, might be taken up by vascular smooth muscle cells, particularly in the presence of glymphatic stasis (caused by reduced arterial pulsation) that could facilitate protein misfolding and aggregation. This is one mechanism by which Aβ might accumulate in the peri-arterial space, as seen in CAA, and the resulting Aβ accumulation might block peri-vascular pathways, further reducing glymphatic clearance. In Aqp4 knockout mice, interstitial clearance is reduced by about 70%, resulting in a 55–65% reduction in Aβ clearance. In AD, AQP4 expression could be decreased, given that in cultured mouse cortical astrocytes, interstitial Aβ1–42 reduces AQP4 expression, which can lead to additional accumulation of plaque-forming Aβ1–42.In traumatic brain injury (TBI)—a risk factor for AD— reactive gliosis is increased. Initially, AQP4 expression is increased in TBI, but long-lasting AQP4 mis-localization from peri-vascular end feet to the astrocytic soma occurs, resulting in reduced peri-vascular AQP4 availability, which can reduce Aβ clearance. Both TBI and AD are associated with peri-vascular inflammation, and these changes might partly explain the link between these conditions. In mice, Aβ clearance during sleep is twice as fast as during awake periods. This increase in Aβ elimination is mediated by a 60% increase in the volume of the extracellular space, which might be modulated by a change in astrocyte cell volume in response to change in adrenergic signalling, as would be expected during sleep. This expansion of the extracellular space was caused by sleep itself rather than circadian rhythms, as it not only occurred during normal sleep, but could also be induced with anaesthesia. Circadian rhythm disturbances have been reported in patients with AD, and might affect clearance through a different mechanism involving increased oxidative stress caused by decreased expression of circadian clock genes, which are involved in protection from oxidative damage. Study describing the glymphatic system demonstrated that accelerated ISF-to-CSF bulk flow was partly responsible for the increase in total Aβ clearance during sleep, representing about 40% of total clearance, which can be calculated from the clearance rate constant data. 60% is probably attributable to accelerated BBB transport of Aβ, because during these transport clearance measurements, the degradation of AB was minimal, which is in line with previous reports. This finding might result from the glymphatic system flushing Aβ toward the BBB during sleep. Thus, sleep could indirectly increase BBB clearance of Aβ through increased glymphatic bulk flow, but it might also directly increase clearance through the BBB via various mechanisms, such as molecular changes (for example, upregulated LRP1), as seen with AD-protective physical and cognitive activity in mice.These findings might partly explain why sleep impairment increases the risk of AD.
Cerebro-spinal fluid absorption clearance Aβ in the circulating CSF can be absorbed either through the arachnoid villi and BCSFB into the circulation, or through the peri-vascular and perineural spaces— and possibly the meningeal lymphatics18—into the lymphatic system.CSF absorption clearance of Aβ by the circulatory and lymphatic systems depends on CSF production, BCSFB integrity and transporters, arachnoid villi resistance, and lymphatic absorption of the CSF. In ageing and AD, these factors are impaired in a number of ways. In ageing, and particularly in AD, CSF production by the choroid plexus is reduced, as shown by decreased water secretion into the ventricles via AQP1 water channels. In AD, the choroid plexus undergoes many structural changes, such as calcification, fibrosis and Aβ deposition, all of which can obstruct CSF production. These structural changes affect BCSFB integrity, thereby reducing Aβ clearance. Many of the Aβ transporters expressed at the BBB, including LRP1, LRP2, ABCB1 and RAGE, are also found at the BCSFB. LRP1 is likely to have an important role in ventricular Aβ clearance at the BCSFB, given that the overall clearance rate of Aβ from the CSF is fivefold faster than the rate observed via CSF flow through the arachnoid villi. The age-related change in expression of many BCSFB transporters follows an opposite pattern to that observed at the BBB for Aβ, such that there is increased efflux and decreased influx- transporter expression, which is suggested to be a result of the BCSFB compensating for age-dependent BBB transporter defects. CSF outflow resistance at the arachnoid villi is increased in AD. This increased resistance is mechanistically similar to normal pressure hydrocephalus, and has been proposed to result from amyloid deposition and fibrosis at the arachnoid villi, resulting in decreased CSF bulk outflow and, thus, decreased CSF Aβ absorption into the blood. Although no evidence has yet been obtained that CSF Aβ levels initially increase in LOAD, reduced CSF turnover would be expected to result in heavily Aβ-laden recirculating CSF, subsequently resulting in reduced concentration as Aβ is deposited in plaques, in the vasculature as CAA, and in the meninges, thereby increasing outflow resistance at the arachnoid villi.
Lymphatic absorption of CSF decreases with age—the primary risk factor for LOAD. In EOAD, by contrast, overproduction of Aβ might result in increased absorption of Aβ by the lymphatic system, as demonstrated in a transgenic mouse model of AD, in which increasing Aβ levels in cervical and axillary lymph nodes mirrored increased Aβ levels in the brain. Clearance of tauTau—a splicing variant of the microtubule- associated protein tau (MAPT)—is an intra-cellular neuronal protein that stabilizes axons. Intra-cellular tau (i-tau) can undergo 2 transformations that are relevant to its clearance: modification and release. Tau modification is regulated by phosphorylation. I-Tau can undergo non degradative cleavage by proteolytic enzymes, such as amino-peptidases, thrombin, HTRA1, calpain and caspases. These enzymes produce proteolytic fragments, which can ultimately be degraded; these fragments have an increased propensity to form aggregates, resulting in reduced clearance. In AD, i-tau is hyper- phosphorylated, which induces the formation of insoluble NFTs that cannot readily be cleared, and can also be neurotoxic. Neuronal activation (presynaptic glutamate release), neuronal death and increased i-tau concentration or aggregation trigger the release of i-tau into the extracellular space, leading to elevated CSF tau levels. Transporters that specifically transport tau through the BBB have not been identified, which suggests that tau does not undergo clearance through the BBB, except after brain injury, when BBB permeability is temporarily increased. Tau is thought to be cleared from the brain primarily by degradation, ISF bulk flow, and CSF absorption clearance. Recent studies using passive immunization with anti-tau oligomer antibodies have shown that like Aβ, pathological tau can be cleared fom the brain by a peripheral sink mechanism, indicating that enhancement of tau clearance might be a therapeutic strategy in AD. Degradation clearance Tau is mainly cleared through intra-cellular degradation by lysosomes via the autophagy–lysosome pathway, and by proteasomes via the ubiquitin– proteasome pathway. AD-related dysfunction of these pathways has been suggested to result in the accumulation of soluble i-tau. I-Tau can also be degraded by proteases (caspases) in response to apoptosis- inducing stressors, and by calpain in response to elevated intra-cellular calcium concentrations. Phosphorylation of tau by protein kinase A increases its resistance to degradation by calpain; AD-associated hyper-phosphorylation of tau has been suggested to impair tau turnover and result in tau accumulation in the form of NFTs. Following release of i-tau into the extracellular space—a process that could result from neuronal death or stimulation—e-tau can be internalized by other neurons via endocytosis, leading to prion-like spreading of tau pathology. Soluble e-tau might bind to muscarinic type 1 and type 3 receptors, thereby increasing intra-cellular calcium levels, which might facilitate further release of i-tau. Tau released into the extracellular space is highly stable: its CSF half-life is 12–14 h, compared with about 2 h for Aβ.Interstitial fluid bulk-flow clearance. If e-tau is not cleared by endocytosis, it might be cleared via the glymphatic system. Following TBI, glymphatic clearance of ISF solutes was impaired by about 60% in wild-type mice, and to an even greater extent in Aqp4 knockout mice that displayed NFTs, neuro-inflammatory reactive gliosis, and neuro-degeneration. These findings support the link between TBI and tau aggregation, with resulting neuro-degeneration similar to that seen in AD and chronic traumatic encephalopathy. Recirculation of CSF poses an additional challenge to tau clearance: cells closest to the peri-arterial boundary might internalize tau from the tau-laden recirculating CSF within the peri-arterial space. Cerebro-spinal fluid absorption clearance as for any soluble substance in circulating CSF, tau can be absorbed either into the circulatory system from the arachnoid villi and BCSFB, or from the lymphatic system through the peri-vascular and peri-neural spaces. Meningeal lymphatic vessels provide another possible route, although their specific contribution to tau elimination has not been tested [23].
Susan J Thompson, et al: The data clearly indicate that freezing the spinal meninges markedly increases the in vitro permeability of morphine when compared to fresh tissue. Of note, the value reported for morphine permeability through fresh tissue in these experiments is identical to that reported in earlier studies from this laboratory (0.64 +/− 0.17 cm/min x 10-3)2, indicating that the model is inherently reproducible. The principal barrier to diffusion in the meninges has been shown histologically and functionally to be within the arachnoid layer. The arachnoid contains a barrier cell layer with frequent tight junctions that effectively seal plasma membranes of adjoining cells. Stripping of the arachnoid layer in M nemestrina increases the meningeal permeability of opiates nearly 10-fold. In cadaveric frozen tissue, cell death and/or tissue disruption, caused by ice crystal formation might certainly alter the microanatomy of the arachnoid barrier cell layer and cause a substantial increase in the apparent permeability of epidural drugs. The results of this study may also explain some seeming inconsistencies in the literature regarding the barrier properties of the spinal meninges. For example, Moore et al., using previously frozen cadaveric human tissues, concluded that molecular weight was the principle determinant of meningeal permeability. In contrast, using the model described in this study, previous reports from this laboratory showed that molecular weight plays no role in determining meningeal permeability. Moore et al. reported the permeability coefficient for morphine to be 3.6 cm/minx10-3, which is nearly six fold greater than the permeability coefficient for morphine through fresh primate meningeal tissue in this and previous studies from our laboratory. The permeability coefficient reported by Moore et al. is nearly identical to that reported herein through frozen tissue (3.2 cm/minx10-3). The likely explanation for these differences is the use of frozen tissue by Moore et al. McEllistrem et al. concluded that there was little difference in meningeal permeability among the opioids and local anesthetics they tested using previously frozen cadaveric human tissues. In contrast, we have previously shown that there are clear differences in the permeability characteristics of the same drugs when studied in fresh tissues maintained in a viable state. The difference in tissue preparation probably explains the seeming incongruity. Studies of meningeal permeability and the factors governing it might best be performed in vitro, where uncontrollable variables such as vascular uptake, drug absorption into epidural fat, and others are not a factor.in vitro studies are only useful to the extent that the tissue being investigated behaves as it does in vivo. The results of this study suggest that frozen meningeal tissues do not behave as they would in vivo and are inappropriate for studies of drug permeability [24].
Jason Weller, et al: Anti-amyloid, according to the amyloid cascade hypothesis, toxic plaques are the earliest manifestation of disease, a statement supported by evidence of Aβ up to 20 years prior to the onset of symptoms. Researchers found in 2013 that this abnormal amyloid plaque induces the phosphorylation of tau protein, which then spreads almost infectiously via microtubule transport to neighboring neurons, leading to neuronal death. One class of medications developed using this evidence is the monoclonal antibodies (passive immunotherapy). This type of treatment involves injection of an antibody that targets abnormal Aβ and facilitates its removal from the brain. Two such monoclonal antibodies were initially developed in 2014 to remove these plaques from the brains of people with AD38,39. Neither medication improved cognitive scores in patients with mild-to-moderate disease (MMSE 16–26), leading researchers to conclude that these medications may show benefit only when administered in the early stages of MCI and mild dementia. However, a new study regarding the effect of this class of medication in patients with few to no symptoms (MMSE 20–26) but a positive amyloid PET imaging result also failed to show a significant difference in cognitive outcomes between the study group and asymptomatic controls40. Studies involving similar drugs in this class are ongoing, with the goal of improving or preserving cognition in patients with MCI due to AD. Another approach to decreasing Aβ plaque burden in the brain is the inhibition of the enzymes that produce the Aβ peptide from its precursor, amyloid precursor protein (APP). Currently, multiple drugs are in development which target β-site APP cleaving enzyme 1 (BACE1), which is thought to be essential for the production of Aβ peptides41. Though previous studies of BACE1 inhibitors failed to yield meaningful results in human subjects, the novel agent verubecestat recently achieved a more than 40-fold reduction in Aβ levels in the brains of rodents and primates, and it has shown a good safety profile in early human trials. Currently, another drug is under investigation for its effect on memory and cognitive function in older patients with positive biomarkers or family history of AD, known as the EARLY study. Researchers showed in 2014 that combination therapy with a monoclonal antibody and a BACE1 inhibitor significantly reduced the amount of Aβ in amyloid-producing mice. While there are no current trials underway utilizing this approach in humans, many experts believe that combination therapy employing both approaches to eliminate Aβ will ultimately lead to success in AD treatment. Since p-tau appears to be the downstream pathology and is likely the direct cause of symptoms in AD, drugs to reduce the burden of this protein are also in development. Many different tau vaccines have shown both safety and efficacy in animal models, and, in one recent small study, an anti-tau drug demonstrated a good safety profile and even stimulated a positive immune response in human patients. Several other early phase trials of drugs which target the tau protein are currently underway, though results are yet to be published. Neural circuitry: The failure of some targeted therapies toward Aβ in large-scale clinical trials has led to the hypothesis that, although the abnormal protein is implicated at the onset of AD, the progression of clinical symptoms is due to more global neural network dysfunction. Gamma oscillation, a high-frequency brainwave rhythm, is associated with inter-neuronal communication in virtually all brain networks and may help to distinguish between true and false memories. Recently, researchers at the Massachusetts Institute of Technology found that induction of gamma-frequency oscillations led to reduced Aβ deposition and improved cognitive outcomes in an AD mouse model. This was done by using a non-invasive 40 Hz photic stimulator to entrain the desired frequency in the mouse cortex. This method is also currently in early phase trials in humans, utilizing both visual and auditory stimulation [25].
Nalivaeva NN, et al: Targeting the amyloid-β (Aβ) peptide cascade has been at the heart of therapeutic developments in Alzheimer’s disease (AD) research for more than 25 years, yet no successful drugs have reached the marketplace based on this hypothesis. Nevertheless, the genetic and other evidence remains strong, if not overwhelming, that Aβ is central to the disease process. Most attention has focused on the biosynthesis of Aβ from its precursor protein through the successive actions of the β- and γ-secretases leading to the development of inhibitors of these membrane proteases. However, the levels of Aβ are maintained through a balance of its biosynthesis and clearance, which occurs both through further proteolysis by a family of amyloid-degrading enzymes (ADEs) and by a variety of transport processes. The development of late-onset AD appears to arise from a failure of these clearance mechanisms rather than by overproduction of the peptide. This review focuses on the nature of these clearance mechanisms, particularly the various proteases known to be involved, and their regulation and potential as therapeutic targets in AD drug development. The majority of the ADEs are zinc metalloproteases [e.g., the neprilysin (NEP) family, insulin-degrading enzyme, and angiotensin converting enzymes (ACE)]. Strategies for up-regulating the expression and activity of these enzymes, such as genetic, epigenetic, stem cell technology, and other pharmacological approaches, will be highlighted. Modifiable physiological mechanisms affecting the efficiency of Aβ clearance, including brain perfusion, obesity, diabetes, and sleep, will also be outlined. These new insights provide optimism for future therapeutic developments in AD research [26].
Ahmad MH, et al: Alzheimer’s disease (AD), the most common progressive neurodegenerative disorder is characterized by the formation of extracellular amyloid plaques and intra-cellular neurofibrillary tangles (NFTs). Increasing evidences suggest a link between neuroinflammation and neuronal dysfunction in AD, orchestrated by the progressive activation of microglial cells and astrocytes with the consequent overproduction of proinflammatory molecules. The concomitant release of anti-inflammatory mediators antagonizes the inflammatory processes and leading to the severity of the AD pathology. The simultaneous detection of these inflammatory molecules in the pre-symptomatic stage may help in the early diagnosis of the AD. We have discussed the impact of microglia and astrocytic cells, the principal agents in the neuroinflammation process, in relation to the progression of the AD. Modulation of the risk factors and targeting of these immune mechanisms could lead to better therapeutic or preventive strategies for the AD. Further studies need to determine, how the inhibition of inflammatory factors could be used for the AD alternative therapies [27].
GA Malik, et al: The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. This work provides interim results from a double-blind, placebo- controlled phase 1b randomized trial (PRIME). Its primary outcome was to investigate safety, tolerability, pharmacokinetics, and pharmacodynamics of monthly infusions of aducanumab in patients with prodromal or mild AD with brain Aβ pathology confirmed by molecular positron emission tomography (PET) imaging. 165 participants were randomized across 33 centres to receive monthly infusions of placebo, or 1, 3, 6, or 10 mg/kg doses of aducanumab for 12 months. 40 patients dropped out, leaving between 21 and 32 individuals per group for analysis. The primary outcome was reduction in brain Aβ plaques as measured by florbetapir PET imaging in a dose- and time-dependent fashion at baseline, six months, and 12 months. Additional cognitive tests were also performed but were exploratory as the study was not powered to detect clinical change. At 6 and 12-month intervals there was a significant dose-dependent reduction of Aβ deposits. In the placebo group, the florbetapir standardized average uptake value ratio (SUVR) was 1.44, compared to 1.16 in the 10 mg/kg group. The reduction in plaque load was seen in participants with both prodromal and mild AD, and regardless of ApoE4 carrier status and reduced brain Aβ in a dose- and time-dependent manner. The most common adverse effects were amyloid-related imaging abnormalities (ARIA), headache, urinary tract infection and upper respiratory tract infection. ARIA-vasogenic oedema occurred in no patients in the placebo group but up to 41% of the high dose group. ARIA although not fully understood, generally occurred early in the course of treatment and is likely to be related to increased extracellular fluid, however, no patient were hospitalised for this and there were no drug related deaths. Based on its primary outcomes the PRIME study shows that aducanumab penetrates the brain and decreases Aβ in patients with AD in a time and dose-dependent manner. With only 165 participants the study was not powered to detect clinical change so that the preliminary findings of a slowing of clinical decline measured by Clinical Dementia Rating (Sum of Boxes and Mini Mental State Examination scores versus placebo at 12 months) must be interpreted with some caution. The greatest effect in reducing brain Aβ plaques was observed in the 10 mg/kg group (p<0.05 versus placebo), and whereas significant Aβ reduction was detectable by 6 months, any clinical effects were not seen until 12 months. Analysis also suggested that performance on the CDR-SB and the MMSE stabilized only in patients who had a substantial reduction in amyloid at one year. Patients for whom there was no reduction in imaging correlates of Aβ levels, declined cognitively in a similar pattern to the placebo group.
The authors acknowledged several study limitations of the PRIME phase 1b study, including staggered parallel-group design, small sample sizes, limited region (USA only), and possible partial un-blinding due to the imaging frequency required following ARIA. However, this study suggests that a reduction of brain Aβ may confer a clinical benefit, thereby supporting the amyloid hypothesis and providing impetus to research in this field, including unravelling the exact molecular basis of clearance of Aβ clearance from the brain following aducanumab. The results from this Phase 1b study support results from earlier preclinical trials demonstrating aducanumab brain penetration, target engagement, and dose-dependent clearance of Aβ plaques, and if confirmed in future studies powered to detect clinical benefit studies would provide support for aducanumab as an Aβ-removing, disease-modifying therapy for AD. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomized, controlled, double-blind, parallel-arm, phase 3 trial. Based on the previous potential efficacy of methylthioninium chloride in patients with AD, a modified stable reduced form of the methylthioninium moiety was identified as a potential therapeutic agent. Leuco-methylthioninium bishydromethanesulfonate (LMTM) acts as a selective inhibitor of tau protein aggregation both in vitro and in transgenic mouse models. The aim of this study was to determine whether LMTM was safe and effective in modifying disease progression in patients with mild to moderate AD. The primary outcome measure was progression on the Alzheimer’s disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and the AD Co-operative Study-Activities of Daily Living Inventory (ADCS-ADL) scales from baseline, assessed at week 65 in the modified intention-to-treat population.
This was a large, multinational trial of 15 months duration. It was a randomized, controlled, double-blind, parallel-group trial involving 115 academic centres and private research clinics in 16 countries in Europe, North America, Asia, and Russia involving patients younger than 90 years with mild to moderate Alzheimer’s disease. Patients taking other medicines for AD were not excluded as the study group felt it infeasible not to allow their inclusion. Participants were randomly assigned (3:3:4) oral tablets to 75 mg LMTM twice a day, 125 mg LMTM twice a day, or control (4 mg LMTM twice a day to maintain blinding with respect to urine or faecal discolouration). Randomisation was achieved via an interactive web response system and all participants and all assessors were masked to treatment assignment throughout the study. In total 891 participants were assigned to treatment (357 to control, 268–75 mg LMTM twice a day, and 266–125 mg LMTM twice a day). Neither co-primary outcomes demonstrated treatment benefit at any dose: ADAS-Cog score compared with control (n=354, 6.32, 95% CI 5.31–7.34): 75 mg LMTM twice a day (n=257)−0.02, −1.60 to 1.56, p=0.9834, 125 mg LMTM twice a day (n=250) −0.43, −2.06 to 1.20, p=0.9323 and change in ADCS-ADL score compared with control (−8.22, 95% CI −9.63 to −6.82): 75 mg LMTM twice a day −0.93, −3.12 to 1.26, p=0.8659; 125 mg LMTM twice a day −0.34, −2.61 to 1.93, p = 0.9479). The most common adverse events were gastrointestinal and urinary effects with both high doses of LMTM, and were the commonest causes for discontinuation. Non-clinically significant dose-dependent reductions in haemoglobin concentrations were the most commonly identified laboratory abnormality. Amyloid-related imaging abnormalities were noted in less than 1% (8/885) of participants. As with previous study’s targeting tau protein accumulation analysis of the primary outcomes for this study was negative and the results do not suggest benefit of LMTM as an add-on treatment for patients with mild to moderate Alzheimer’s disease. Findings from a recently completed 18-month trial of patients with mild AD are to be released in the near future [28].
Eric P Thelin, et al: Neuro-intensive care following traumatic brain injury (TBI) is focused on preventing secondary insults that may lead to irreversible brain damage. Microdialysis (MD) is used to detect deranged cerebral metabolism. The clinical usefulness of the MD is dependent on the regional localization of the MD catheter. The aim of this study was to analyze a new method of continuous cerebro-spinal fluid (CSF) monitoring using the MD technique. The method was validated using conventional laboratory analysis of CSF samples. MD-CSF and regional MD-Brain samples were correlated to patient outcome. A total of 14 patients suffering from severe TBI were analyzed. They were monitored using (1) a MD catheter (CMA64-iView, nD7448 MD samples) located in a CSF-pump connected to the ventricular drain and (2) an intra-parenchymal MD catheter (CMA70, nD8358 MD samples). CSF-lactate and CSF-glucose levels were monitored and were compared to MD-CSF samples. MD-CSF and MD-Brain parameters were correlated to favorable (Glasgow Outcome Score extended, GOSe 6–8) and unfavorable (GOSe 1–5) outcome. Results: Levels of glucose and lactate acquired with the CSF-MD technique could be correlated to conventional levels. The median MD recovery using the CMA64 catheter in CSF was 0.98 and 0.97 for glucose and lactate, respectively. Median MD-CSF (CMA 64) lactate (p D0.0057) and pyruvate (p D0.0011) levels were significantly lower in the favorable outcome group compared to the unfavorable group. No significant difference in outcome was found using the lactate: pyruvate ratio (LPR), or any of the regional MD-Brain monitoring in our analyzed cohort. Conclusion: This new technique of global MD-CSF monitoring correlates with conventional CSF levels of glucose and lactate, and theMDrecovery is higher than previously described. Increase in lactate and pyruvate, without any effect on the LPR, correlates to unfavorable outcome, perhaps related to the presence of erythrocytes in the CSF [29].
Ågren-Wilsson, et al: In idiopathic adult hydrocephalus syndrome (IAHS), a pathophysiological model of “chronic ischaemia” caused by an arteriosclerotic process in association with a CSF hydrodynamic disturbance has been proposed. To investigate whether CSF hydrodynamic manipulation has an impact on biochemical markers related to ischaemia, brain tissue oxygen tension (PtiO2), and intracranial pressure. A microdialysis catheter, a PtiO2 probe, and an intracerebral pressure catheter were inserted into the periventricular white matter 0–7 mm from the right frontal horn in 10 patients with IAHS. A subcutaneous microdialysis probe was used as reference. Intracranial pressure and intracerebral PtiO2 were recorded continuously. Samples were collected for analysis between 2 and 4 pm on day 1 (baseline) and at the same time on day 2, two to four hours after a lumbar CSF hydrodynamic manipulation. The concentrations of glucose, lactate, pyruvate, and glutamate on day 1 and 2 were compared. Results: After CSF drainage, there was a significant rise in the intracerebral concentration of lactate and pyruvate. The lactate to pyruvate ratio was increased and remained unchanged after drainage. There was a trend towards a lowering of glucose and glutamate. Mean intracerebral PtiO2 was higher on day 2 than on day 1 in six of eight patients. There is increased glucose metabolism after CSF drainage, as expected in a situation of postischaemic recovery. These new invasive techniques are promising tools in the future study of the pathophysiological processes in IAHS [30].
Alexander V Shulyakov, et al: This study was undertaken to determine if dialysis of damaged brain surface can reduce cerebro-spinal fluid (CSF) pressure and progressive brain edema. The authors secondarily determined if local brain cooling was simultaneously possible. Telemetric pressure transmitters were implanted into the lumbar subarachnoid space of 58 young adult male rats. Cryogenic brain injury was created and 2 hours later decompressive craniectomy was performed. An osmotic cell with a semipermeable dialysis membrane placed on the damaged brain surface was perfused with dextran 15% solution for 2 or 4 hours. Water content was determined in the cerebral hemispheres using the wet-dry weight method. Evans blue–albumin spread was measured morphometrically. Brain temperature was measured bilaterally. The CSF pressure increased after cryogenic injury and decreased after cranio-tomy. Two hours of brain dialysis significantly reduced CSF pressure in comparison with craniotomy alone and sham dialysis. Injured brain had higher water content, but this was not affected by dialysis. Spread of Evans blue–albumin, was significantly reduced by the treatment. Cooling of the dialysis solution caused significant local brain cooling. Surface dialysis of cryogenically injured rat brain controls CSF pressure and reduces intra-parenchymal spread of edema fluid in the acute period after injury. The authors postulate that edema fluid moves into the osmotic cell rather than spreading through the uninjured brain. Long-term experiments will be needed to prove that this combination therapy is effective [31].
Fahima Mayer, et al: Pharmacologic remedy of many brain diseases is difficult because of the powerful drug exclusion properties of the blood-brain barrier (BBB). Chemical isolation of the vertebrate brain is achieved through the highly integrated, anatomically compact and functionally overlapping chemical isolation processes of the BBB. These include functions that need to be coordinated between tight diffusion junctions and unidirectional-acting xenobiotic transporters. Understanding of many of these processes has been hampered, as they are not well mimicked by ex vivo models of the BBB and have been experimentally difficult and expensive to disentangle in intact rodent models. Here we show that the Drosophila melanogaster (Dm) humoral/CNS barrier conserves the xenobiotic exclusion properties found in the vertebrate vascular endothelium. We characterize a fly ABC transporter, Mdr65, that functions similar to mammalian xenobiotic BBB transporters and show that varying its levels solely in the Dm BBB changes the inherent sensitivity of the barrier to cytotoxic pharmaceuticals. We demonstrate orthologous function between Mdr65 and vertebrate ABC transporters by rescuing chemical protection of the Dm brain with human MDR1/Pgp. These data indicate that the ancient origins of CNS chemoprotection extend to both conserved molecular means and functionally analogous anatomic spaces that together promote CNS selective drug partition. Thus, Dm presents an experimentally tractable system for analyzing physiological properties of the BBB in an intact organism [32].
David Bueno, et al: Within the consolidated field of evolutionary development, there is emerging research on evolutionary aspects of central nervous system development and its implications for adult brain structure and function, including behavior. The central nervous system is one of the most intriguing systems in complex metazoans, as it controls all body and mind functions. Its failure is responsible for a number of severe and largely incurable diseases, including neurological and neurodegenerative ones. Moreover, the evolution of the nervous system is thought to be a critical step in the adaptive radiation of vertebrates. Brain formation is initiated early during development. Most embryological, genetic and evolutionary studies have focused on brain neurogenesis and regionalization, including the formation and function of organising centers, and the comparison of homolog gene expression and function among model organisms from different taxa. The architecture of the vertebrate brain primordium also reveals the existence of connected internal cavities, the cephalic vesicles, which in fetuses and adults become the ventricular system of the brain. During embryonic and fetal development, brain cavities and ventricles are filled with a complex, protein-rich fluid called cerebrospinal fluid (CSF). However, CSF has not been widely analysed from either an embryological or evolutionary perspective. Recently, it has been demonstrated in higher vertebrates that embryonic cerebrospinal fluid has key functions in delivering diffusible signals and nutrients to the developing brain, thus contributing to the proliferation, differentiation and survival of neural progenitor cells, and to the expansion and patterning of the brain. It has been shown that the composition and homeostasis of CSF are tightly controlled in a time-dependent manner from the closure of the anterior neuropore, just before the initiation of primary neurogenesis, up to the formation of functional choroid plexuses. We draw together existing literature about the formation, function and homeostatic regulation of embryonic cerebrospinal fluid, from the closure of the anterior neuropore to the formation of functional fetal choroid plexuses, from an evolutionary perspective. The relevance of these processes to the normal functions and diseases of adult brain will also be discussed [33,34].
Experimental project hypotesys: in vitro
In order to verify the efficacy and safety of a SPINAL CORD neuro tissue (or LCR) dialitic like - depurative procedure is possible to Hypotize an in vitro model (animal model?) whit a spine cord to be submitted to the procedure, using different kind of solution or other (lime MD) to depurate form toxic substanties responsible of progression of some neuro-degenerative disease. The procedure must be observed in Different time of exposition and in different level of action to find the really best condition. (Since also after acute trauma). The right window of time to have the best result must be evalued. The same must be verified the better direction that can provide useful effect (SPINAL CORD top- bottom or bottom – top) and if a pharmacological or a non- pharmacological system (MD or other physic process) can provide better results. The affinity of innovative pharmacological molecule must be evalued to choose the one with high link with the toxic substantia (amyloids, proteins, amminoacids, FREE oxygen radicals and other), with the best profile for an efficacy tissue celaracence. Other parameter must be taken in consideration like: lipophilic- idorfilic profile of toxic substantia, molecular weight, molecular conformation, links profile, electrical charge, Idrogen binds, and other useful to have an efficacy extraction. Safety of the procedure must be devalued (histology, electric conduction and other possible).
Form literature reported is possible to verifay that
A) Reduced CSF turnover may be a contributing factor to the buildup of toxic metabolites and proteins that cause neuro-degenerative disorders.” And that “reduced CSF turnover may be a contributing factor to the buildup of toxic metabolites and proteins that cause neuro-degenerative disorders”. https://news.psu.edu/story/579374/2019/07/01/research/sense-smell-pollution-and-neurological-disease-connection-explored UNIVERSITY PARK.
B) A team of neuroscientists at the University of Rochester Medical Center has identified a fascinating fast-track cleansing system in the brain called the glymphatic system”. Is reported in the article DETOXING FOR BRAIN HEALTH – NEW RESEARCH FINDINGS: CranioSacral Therapy Improves Glymphatic Cleansing of Brain Tissue Carolyn Simon
C) In most of the body, a network of vessels carry lymph, a fluid that removes excess plasma, dead blood cells, debris and other waste. But the brain is different. Instead of lymph, the brain is bathed in cerebro-spinal fluid” and “To see how waste is carried by cerebro-spinal fluid in a living mouse, they injected the mice with radioactive molecules that could be traced using laser-scanning technology. The molecules’ journey began after being injected into the subarachnoid space, a cavity between membranes covering the brain and spinal cord. The researchers observed that, like a river, cerebro-spinal fluid carried these molecules rapidly along specific channels. Glial cells along the outside of arteries form these channels, creating a flume for cerebro-spinal fluid that follows the brain’s blood vessels. In addition, the researchers found that these glial cells mediate the channel’s activity, assisting the flow of fluid through the channel. From channels alongside arteries, the tracer-bearing fluid then passes through brain tissues. At the other end of tissues, it flows into similar channels along veins. The fluid follows these veins then either returns to the subarachnoid space, enters the bloodstream or eventually drains into the body’s lymphatic system”. “The researchers injected proteins called amyloid beta into mice’s brains. In Alzheimer’s, this protein—present in all healthy brains—can accumulate and clump, developing into cell-damaging plaque. The researchers compared mice with a normal glymphatic system to those with a disabled gene that prevented glial cells from assisting in the fluid flow. They found that in the normal mice, the protein rapidly cleared from the brain along these channels, but amyloid removal diminished in the gene-altered animals.
D) Each day, the adult brain eliminates a quarter of an ounce of worn-out proteins that must be replaced with newly made ones, a figure that translates into the replacement of half a pound of detritus a month and three pounds, the brain’s own weight, over the course of a year. To survive, the brain must have some way of flushing out debris” and “Experiments that we conducted in mice showed that during sleep, the glymphatic system did indeed remove beta-amyloid from the brain with remarkable efficiency: its clearance rate more than doubled with sleep.
E) The primary traumatic mechanical injury to the spinal cord causes death of a number of neurons. These events are then exacerbated by a variety of secondary mechanisms including vascular changes, ischemia, vasospasms, hemorrhage and thrombosis, neuro-transmitter (especially glutamate) accumulation, generation of free radicals and nitric oxide (NO), calcium overload, compromised energy metabolism and inflammatory factors. According article J. Pineal Res. 2010; 49:201–209 Melatonin plus exercise-based neurorehabilitative therapy for spinal cord injury.
F) Current research suggests that an impaired clearance in late onset AD plays a critical role in the process of amyoid formation and the pathogensis of the disease. Many pathways are currently being investigated, among them Peri-vascular drainage, receptor-mediated celluptake, blood Brain barrier (BBB) transport and local proteolytic degradation, All undoubtedly contributors to brain Ab clearance in Conjunction with the bulk flow of ISF into the CSF through the Choroid plexus epithelium which Remarkably shares many of The receptors involved in BBB clearance as well as the recently described paths for CSF recycling through the ISF.
G) It is known that Alzheimer’s disease primarily affects parts of the brain that play a role in memory, whereas Parkinson’s disease predominantly affects parts of the brain that are involved in body movement. The reasons that other brain regions remain unaffected in these diseases are unknown.
H) Reduction of the CSF turnover rate during ageing leads to accumulation of catabolites in the brain and CSF that are also observed in certain neuro-degenerative diseases.
I) Extracellular Aβ deposits can be removed from the brain by various clearance systems, most importantly, transport across the blood–brain barrier.
J) Increasing evidences suggest a link between neuroinflammation and neuronal dysfunction in AD, orchestrated by the progressive activation of microglial cells and astrocytes with the consequent overproduction of proinflammatory molecules”. AHMAD
K) The present study shows that treatment with detox gel in AD mice can be effective as a therapeutic method for lowering Aβ levels in the APPSWE mice (Tg2546). The high affinity sequestering ability combined with the lack of immunogenicity and capacity to improve memory parameters make it an interesting drug candidate for the treatment of AD.
Thus our detox gel therapy might become a welcome non-immune based therapy with a future in human clinical settings” and “Quantitative analysis of Aβ-42 in brain Aβ in brain was extracted by methods described in experimental methods section. Aβ-42 levels were measured in brain extracts by an ELISA using a commercial antibody. The twostep extraction method that we employed yields total Aβ. The results show that there is a reduction in the level of total Aβ-42 by 30% in the group of mice that received the detox gel when compared to the untreated group with a statistical significance (p<0.001)” novel Detox Gel Depot sequesters β-Amyloid Peptides in a mouse model of Alzheimer’s Disease, Ranjini K. Sundaram et al. [35] and related the Brain’s Drain: Neuroscientists Discover Cranial Cleansing System. The time of BBB evolution is unknown, but through ancient fish BBB studies Bundgaard and Abbott (2008) : invertebrates are descendants of a common ancestor that had evolved a glial cell based BBB before differentiation and six separate convergent evolutions of the endothelial. They support this assumption by observations made on ancient species that still exist today (lung fish, hag fish, lamprey) and their tendency to retain glial cell based BBBs. evidence that this was the first type of BBB, by pointing out that many mammals for a primitive glial cell BBB in the womb before the more complex endothelial cell BBB is formed, and many vertebrates and invertebrates that form a endothelial BBB retain sections of BBB made up of more permeable glial cells (Abbott 2005). The reason for its presence is normal brain- function requires a large amount energy (to supply enough oxygen and remove adequate amounts of wastes for respiration), large networks of capillaries must be constructed in the brain for molecular exchange. A high prevalence of capillaries not only allows for quick diffusion of reactants and products of respiration, increases the possibility that other contents in the blood may diffuse across the capillary wall. The BBB evolved to allow for a higher rate of selectivity for what may pass through to the brain tissue to help preserve /protect the brain from possible detrimental molecules or organisms in the blood.
Related the BRAIN EFFLUX status (physiology, BEE barrier et other factors) and SNC clearance is clearly that some depurative procedure are currently applied to some poisoning or disease (in example in severe or terminal renal failure). But if this procedure present various level of efficacy this kind of approach is not currently applied in other pathology like some neuro-degenerative spinal cord disease. (ALS involved with SOD mutation) but also in some spinal cord traumatic condition with accumulation of amino acid after ischemia. An informatics approach help in adeguately set the problem to increase efficacy but reducing the risk of damage for a delicate structure. The algoritm: need to detox the micro-environment and related detoxicant strategy (OR modifying the mebabolic catabolic status) imply a not toxicity of the procedure versus the complex and weakness tissue as the spinal cord. The process must set the way of sub ministration, the mechanism of action, the safety and all over involved in a practical experience. The question is way related blood purification with dialitic procedure is possible to reduce the toxcic urea conc. And none can be used in other tissue? (dialitic procedure to be consider as a separating procedure of toxic from tissue in every way possible: phisic-chemical- pharmaocolgical, MABS, medical device or other strategy) (Table 1). In emodialisys: form ancient dal greck αἷμα, àima, “blood “, e διάλυσις, diàlysis, “separation“, derivate form διαλύω, dialỳō, “distinguish”) is a phisic therapy that replace some renal function in uremic patient in IRA. The procedure replace 4 renal function remove of toxic substantia, electrolitic and acido – bases re balances, liquid remove (in anuric pz). The procedure imply that blood pass trought a filter and solution (with a semi permeabile membrane) by witch pass only the toxic subtantia.
| Table 1: Depurative procedure. | |||||
| In use? | Useful | Difficult procedure | Do be developed? | Danger | |
| Emodialisys | Yes | yes | medium | Already in use | accepted |
| Peritoneal dialisys | yes | yes | medium | Already in use | accepted |
| Emoperfusion | yes | yes | yes | Already in use | accepted |
| Plasma exchange | yes | yes | Medium | Already in use | accepted |
| Iperbarich Therapy | yes | yes | Medium | Already in use | accepted |
| ECMO ExtraCorporeal Membrane Oxygenatio | Yes | yes | medium | Already in use | accepted |
| Antidotic therapy | yes | yes | No- little-medium | Already in use | accepted |
| Spinal cord – brain depurative strategy | no | It could be | Very difficult | Yes: Hypotesys to be verified | To be verified on field ( in vitro model- other model ) |
| Other | |||||
Related a new procedure to think depurate Brain or spinal cord tissue Possible Mechanism of action must be evaluated:
Immnunological links: mabs
Differential solubility (idrophilic- lipophilic balance)
Molecular weight (differential)
Dimension-diameter of particles
Differential in electrical charge
Chemical: Complexant agent
Enzymatic-remove
Facilitator of excretions: Chemical groups to be linked to increase clearance
Increase factor that improve catabolism or clearance of toxic substantia
Free radicals blockers strategy
Flogosys control (sustained local action)
Temperature level: low level make more permeability of Bee
Gamma-frequency oscillations? In vitro model
Clearance of the system (brain waste flux) status: Kinetics
Transport at the neurovascular unit across the glial barrier and BBB: depends on the solubility, molecular weight and diameter of the protein and other.
Related the bibliography reported in this article a new depurative strategy to treat some spinal cord and brain neuro-degenerative –inflamatory pathology in example ALS SOD mutation and ALZHEIMER D. related can be hypotized procedure similar to a dyalitic LIKE process or other depurative strategy ( in spinal cord place or LCR ). “The wastes of brain metabolism, peroxidation products and glycosylated proteins, accumulate with age-related decreased CSF turnover. Reduction of the CSF turnover rate during ageing leads to accumulation of catabolites in the brain and CSF that are also observed in certain neuro-degenerative diseases [22]. Observing the TURING machine theory is possible to verify that a conceptual map make possible to translate from a language to other that seem not related ( word War second and ENIGMA machine : secret way of communication ). The same is possible to think that an algoritm – machine can traduce some need in response. In example in some neuro-degenerative or inflammatory brain or spinal cord disease a process that can DEPURATE. Form some toxic metabolites or immune-products can delay the progression of some severe disease. Is possible to introduce the hypotesis that a pharmacological, antidotic or medical devices or other physic strategy can help in this setting providing a sustained action during in time or to restore the normal flux of brain wasting system? In an animal model maoue “The results show that there is a reduction in the level of total Aβ-42 by 30% in the group of mice that received the detox gel when compared to the untreated group with a statistical significance (p<0.001)”. Scope of this work is not to produce details of the procedure but that this strategy can be followed. Can a machine better of humans verify all strategy to be used to depurate noble tissue like central nervous system considered so mysterious by the collective meaning? A Computational machine with artificial intelligence can help in choosing the better strategy respecting physiology of this delicate structure ,providing the right information about affinity of a new product to the toxic substantia and related to the kinetics of the elimination process.
This article is produced under a toxicological- pharmacological and pharmaceutical point of view, without any therapeutic or diagnostic intent only to produce new research hypotesys to be submitted to the researcher.
Ethical consideration
This work is produced under the light of actual ethical rules, is an experimental hypotesys of work to be submitted to the researcher for future development.
- Mauro L, Behzad NA, Nilesh MM, Ghulam RM, Ram KS, et al. Amyotrophic Lateral Sclerosis and Endogenous-Esogenous Toxicological Movens: New Model to Verify Other Pharmacological Strategies. J of Pharmacol & Clin Res. 2018; 6: 555690.
- Luisetto M, Almukhtar N, Rafa AY, Ahmadabadi BN, Mashori GR, et al. Role of plants, environmental toxins and physical neurotoxicological factors in Amyotrophic lateral sclerosis, AD and other Neuro-degenerative Diseases.
- Luisetto M, Almukhtar N, Ahmadabadi BN, Hamid GA, Mashori GR, et al. Clinical Pathology & Research Journal Endogenus Toxicology: Modern Physio- Pathological Aspects and Relationship with New Therapeutic Strategies. An Integrative Discipline Incorporating Concepts from different Research Discipline like Biochemistry, Pharmacology and Toxicology. Clin Pathol.
- Alabdali A, Al-Ayadhi L, El-Ansary A. A key role for an impaired detoxification mechanism in the etiology and severity of autism spectrum disorders. Behav Brain Funct. 2014; 10: 14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24776096
- Nedergaard M, Steven A. Brain Drain Goldman Sci Am. 2016; 314: 44–49.
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005; 79: 157-165.
- Lee H, XieL, Yu M, Kang H, Feng T, et al. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 2015; 35: 11034–11044. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26245965
- Xu J, Huang G, Zhang K, Sun F, Xu T, et al. Activation in Astrocytes Contributes to Spinal Cord Ischemic Tolerance Induced by Hyperbaric Oxygen Preconditioning. J Neurotrauma. 2014; 31: 1343–1353. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24716787
- Hodges GR, Watanabe I. Chemical injury of the spinal cord of the rabbit after intracisternal injection of gentamicin: an ultrastructural study. J Neuropathol Exp Neurol. 1980; 39: 452-75. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6894308
- Sundaram RK, Kasinathan C, Stein S, Sundaram P. Detoxification depot for beta-amyloid peptides. Curr Alzheimer Res. 2008; 5: 26-32. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18288928
- Marsala M, Malmberg AB, Yaksh TL. The spinal loop dialysis catheter: characterization of use in the unanesthetized rat. J Neurosci Methods. 1995; 62: 43-53. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8750084
- Hong Y, Palaksha KJ, Park K, Park S, Kim HD, et al. Melatonin plus exercise-based neurorehabilitative therapy for spinal cord injury. J. Pineal Res. 2010; 49: 201–209. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20626592
- Rathore KI, Kerr BJ, Redensek A, López-Vales R, Jeong SY, et al. Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. J Neurosci. 2008; 28: 12736 –12747. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19036966
- Rajiv R. Ratan, Mark Noble. Novel multi-modal strategies to promote brain and spinal cord injury recovery. Stroke. 2009; 40: S130–S132. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19064774
- Goetz T, Romero-Sierra C, Ethier R, Henriksen RN. Modeling of therapeutic dialysis of cerebrospinal fluid by epidural cooling in spinal cord injuries. Journal of Neurotrauma. 1988; 5: 139-150. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3225857
- Freund P, Curt A, Friston K, Thompson A. Tracking Changes following Spinal Cord Injury: Insights from Neuroimaging. Neuroscientist. 2013; 19: 116–128. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22730072
- Raisman G. A promising therapeutic approach to spinal cord repair. J R Soc Med. 2003; 96: 0141-0768. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12782687
- Camandola S, Mattson MP. Brain metabolism in health, aging, and neuro-degeneration. BMC Neurol. 2017; 36: 1474-1492. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27875990
- Farron L.McIntee, PatriziaGiannoni, StevenBlais, GeorgeSommer, Thomas A Neubert, et al. Invivo Differential Brain Clearance and Catabolism of Monomeric and Oligomeric Alzheimer’s Abprotein.
- Walker S. Jackson. Review Selective vulnerability to neuro-degenerative disease: the curious case of Prion Protein. Disease Models & Mechanisms. 2014; 7: 21-29.
- Marsala M, Sorkin LS, Yaksh TL. Transient Spinal Ischemia in Rat: Characterization of Spinal Cord Blood Flow, Extracellular Amino Acid Release, and Concurrent Histopathological Damage. J Cereb Blood Flow Metab. 1994; 14: 604-614. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8014207
- Sakkaa L, Coll G, Chazala J. Anatomy and physiology of cerebro-spinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011; 128: 309-316. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22100360
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015; 11: 457-470. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26195256
- Thompson SJ, Bernards CM. Barrier Properties of the Spinal Meninges Are Markedly Decreased by Freezing Meningeal Tissues.
- Weller J, Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. 2018.
- Nalivaeva NN, Turner AJ. Targeting amyloid clearance in Alzheimer’s disease as a therapeutic strategy. Br J Pharmacol. 2019. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30710367
- Ahmad MH, Fatima M, Mondal AC. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: Rational insights for the therapeutic approaches. J Clin Neurosci. 2019. 59: 6-11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30385170
- Malik GA, Roberts NP. Treatments in Alzheimer’s disease. J Neurol. 2017; 264: 416–418.
- Thelin EP, Nelson DE, Ghatan PH, Bellander BM. Microdialysis monitoring of CSF parameters in severe traumatic brain injury patients: a novel approach. Frontiers in neurology. 2014. 5: 159. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25228896
- Ågren-Wilsson A, Roslin M, Eklund A, Koskinen L, Bergenheim A, et al. Intracerebral microdialysis and CSF hydrodynamics in idiopathic adult hydrocephalus syndrome. J Neurol Neurosurg Psychiatry. 2003; 74: 217–221. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12531954
- Shulyakov AV, Benour M, Del Bigio MR. Surface dialysis after experimental brain injury: modification of edema fluid flow in the rat model Laboratory investigation. 2008; 109.
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, et al. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009; 29: 3538–3550. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19295159
- Bueno D, Garcia-Fernàndez J. Evolutionary development of embryonic cerebrospinal fluid composition and regulation: an open research field with implications for brain development and function. Fluids and Barriers of the CNS. 2016; 13: 5. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26979569
- Park M, Moon WJ. Structural MR Imaging in the Diagnosis of Alzheimer’s disease and Other Neurodegenerative Dementia: Current Imaging Approach and Future Perspectives. J Radiol. 2016; 17: 827-845. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27833399
- Sundaram RK, Kasinathan C, Stein S, Sundaram P. Novel Detox Gel Depot sequesters β-Amyloid Peptides in a mouse model of Alzheimer’s disease. Int J Pept Res Ther. 2012; 18: 99-106.