More Information
Submitted: 18 November 2020 | Approved: 26 November 2020 | Published: 27 November 2020
How to cite this article: Inoue N, Goto S. Post-stroke dizziness of visual-vestibular cortices origin. J Neurosci Neurol Disord. 2020; 4: 075-078.
DOI: 10.29328/journal.jnnd.1001038
Copyright License: © 2020 Inoue N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Dizziness; Cerebral ischemia; Stroke; Visual-vestibular cortices; Cerebral blood flow
Post-stroke dizziness of visual-vestibular cortices origin
Nobuhiro Inoue1* and Satoshi Goto2,3
1Department of Neurosurgery, Kumamoto Neurosurgical Hospital, Kumamoto 860-0811, Japan
2Department of Neurodegenerative Disorders Research, Institute of Biomedical Sciences, Graduate School of Medical Sciences, Tokushima University, Tokushima 770-8503, Japan
3Parkinson’s Disease and Dystonia Research Center, Tokushima University Hospital, Tokushima 770-8503, Japan
*Address for Correspondence: Nobuhiro Inoue, MD, PhD, Director, Department of Neurosurgery, Kumamoto Neurosurgical Hospital, 6-1-21 Honjo, Kumamoto 860-0811, Japan, Tel.: +81-96-372-3911; Fax: +81-96-362-5135; Email: [email protected]
Many patients with chronic cerebrovascular diseases complain “dizziness”, which is a distortion of static gravitational orientation, or an erroneous perception of motion of the sufferer or of the environment. In the vestibular cortical system, the parieto-insular vestibular cortex (PIVC) serves as the core region having the strong interconnections with other vestibular cortical areas and the vestibular brainstem nuclei. By forming the reciprocal inhibitory interactions with the visual cortex (VISC), it also plays a pivotal role in a multisensory mechanism for self-motion perception. In a line of our studies on post-stroke patients, we found that there was a significant decrease in the cerebral blood flow in both the VISC and PIVC in the patients who suffered from dizziness. In this article, we provide a new concept that due to dysfunction of the visual-vestibular interaction loop, low cerebral blood perfusion in the PIVC and VISC might elicit post-stroke dizziness.
The term “dizziness” has been applied to vertigo, lightheadedness, presyncope, anxiety, and general malaise [1-4]. Dizziness is an unpleasant distortion of static gravitational orientation, or an erroneous perception of motion of either the sufferer or environment. It is not a disease entity but rather the outcome of many pathological or physiological processes. Although the number of patients whose dizziness symptoms are unequivocally attributable to stroke is low, considering the large number of stroke patients who report dizziness symptoms, their actual number may be higher [5,6]. In fact, the percentage of the patients who experienced dizziness after cerebral ischemia is beyond to around 40% (38/94) in our department. There has not been demonstrated the objective examinations for the dizziness which bother many patients yet. We have investigated the relationship between dizziness and cerebral blood flow (CBF) especially in the patients of cerebral ischemia. In this article whether the reciprocal inhibitory visual-vestibular interaction relate to control the dizziness in stroke patients was discussed through our results.
Relationship between dizziness and visual-vestibular cortices
The parieto-insular vestibular cortex (PIVC) is located in a region from which vestibular sensations were induced by electrical stimulation with a depth electrode placed in the Sylvian fissure, medial to the primary acoustic cortex [7]. In view of the strong interconnections between the PIVC and other vestibular cortex areas (mainly 3aV and 2) and the vestibular brainstem nuclei, it has been proposed as the core region in the vestibular cortical system [8-10]. The reciprocal inhibitory visual-vestibular interaction (Figure 1A) is a multisensory mechanism for self-motion perception. It facilitates relating the dominant perception of self-motion to the actual input from one of the two sensory modalities, thereby avoiding perceptual ambiguity. When the perception of self-motion is dominated by the vestibular input (acceleration), the visual cortex (VISC) is inhibited by the PIVC (Figure 1B). An observation can thus be interpreted as the vestibular-visual pendant for self-motion perception. In a positron emission tomography study using caloric vestibular irrigation, activation of the PIVC resulted in a significant, bilateral decrease in the regional cerebral blood flow (CBF) in the occipital VISC that includes Brodmann areas 17, 18, and 19 [11]. Doppler sonography detected a reduction in the posterior cerebral artery blood flow velocity during caloric vestibular stimulation [12]. This suggests that deactivation of the VISC benefits the organism during vestibular stimulation since it suppresses visual motion inputs and thereby protects the vestibular system from conflicting visual motion inputs. When the perception of self-motion is dominated by visual inputs (e.g. during constant-speed car motion), the PIVC is inhibited; this suppresses misleading vestibular inputs resulting from involuntary head oscillations during transportation. These inputs are suppressed by deactivation of the vestibular system [13]. Ombergen, et al. [14] reported an increase in VISC- and a decrease in PIVC connectivity upon visual stimulation.
Evidence for a decreased CBF in the vestibular-visual cortices in post-stroke patients
By using the standard xenon-enhanced CT CBF system, we have carried out CBF measurements in multiple brain regions of post-stroke patients. As we documented elsewhere [15-17], the ischemic lesions in our study population were located in infra- and supratentorial areas and not only in the VISC and PIVC, indicating that ischemia in other brain areas may elicit dizziness. From 2 to 6 months after the start of ibudilast therapy, all eleven [16] and nine [17] patients resolved their dizziness respectively.
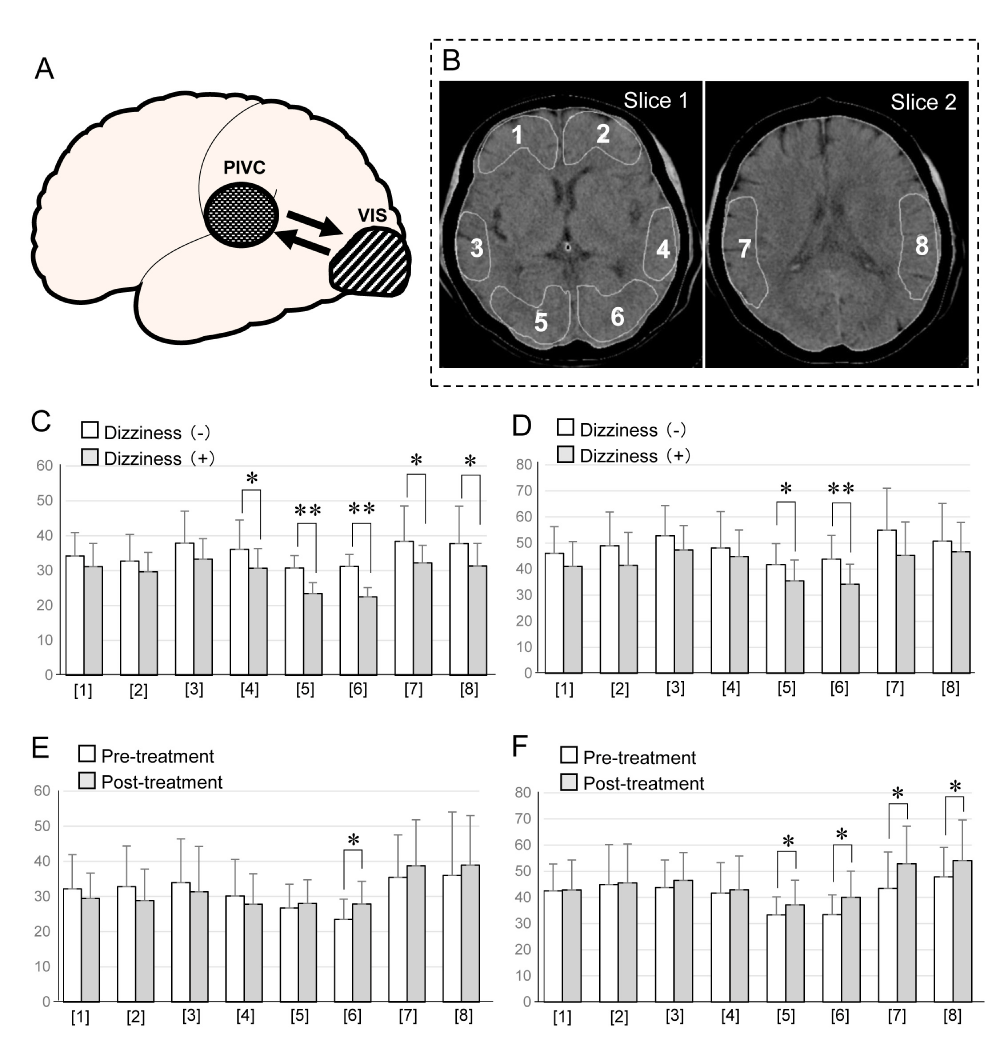
We first compared the CBF in patients with and without dizziness (C). We found that in patients with dizziness, a significant decrease in the CBF was found in both the VISC and PIVC [15], bar graphs of [5],[6],[7] and [8] in figure C implicating both in the elicitation of dizziness. After loading of ACZ, a potent dilator of the cerebral vasculature, the CBF was decreased significantly only in the VISC, bar graphs of [5] and [6] in figure D, suggesting that vasoreactivity in the area of the VISC had not recovered while it was recovered in the PIVC even in patients with dizziness. Based on this observation we posit that inhibition from the PIVC to the VISC might be dominant in the visual-vestibular interaction in patients with dizziness. Deactivation of the VISC during PIVC stimulation suppresses the VISC [18-20]. Therefore, the observed decrease in the CBF in the VISC may be attributable to the selective inhibition of remote excitatory pathways from the PIVC to the VISC, leading to a decrease in the neuronal firing rate in the VISC to below the at-rest level. We think that due to its unrecovered vasoreactivity after ACZ loading, the VISC may be implicated in the elicitation of dizziness.
We next examined the therapeutic effects of ibudilast, which is reportedly known to increase the CBF by facilitating the vasomotor reactivity of cerebral vasculature [16,17], in patients with dizziness. In patients treated with ibudilast, the amelioration of dizziness was accompanied by a significant increase in the CBF in only the VISC, bar graph [6] in figure E. To confirm the presence of an inhibitory effect from the PIVC to the VISC, we recorded the CBF after ACZ loading. We found that post-loading, the CBF was significantly increased in both the VISC and PIVC of patients treated with ibudilast, bar graphs of [5],[6],[7] and [8] in figure F. Therefore, in patients with dizziness, ibudilast administration restored the vasoreactivity of the VISC and PIVC and helped to ameliorate their dizziness. We thus suggest that the reciprocal inhibitory visual-vestibular interaction may be implicated in the manifestation of dizziness in post-stroke patients.
Figure 1: A: Schematic caricature demonstrated the relationship between PIVC and VISC concerned with dizziness in the patients with stroke. PIVC (Parieto Insular Vestibular Cortex), VISC (Visual Cortex).
B: Sections analyzed on CT images. Slice 1 and 2 are axial imaged that include the basal ganglia and temporo-parietal cortex respectively. ROIs were selected on each image. Slice 1: The level of the analyzed slice passed through the basal ganglia and included the midsectin of the anterior horns of the lateral ventricles, the caudate putamen, thalamus, and pineal body posteriorly. Three pairs of cortices corresponding to the frontal cortex ([1] and [2]), the temporal cortex ([3] and [4]), and the occipital cortex ([5] and [6]) = VISC were assessed. Slice 2: [7] and [8] represent the temporo-parietal cortex including the parieto-insular vestibular cortex (PIVC).
C: Comparison of the cerebral blood flow between the patients with dizziness and without dizziness at rest. Blank bar: cerebral blood flow in the Patients without dizziness. Gray bar: cerebral blood flow in the patients with dizziness. Significant difference between without dizziness and with Dizziness (*p < 0.05, **p < 0.01) appeared in [4],[5],[6], and [7],[8].
D: Comparison of the cerebral blood flow between the patients with dizziness and without dizziness after acetazolamide loading. Blank bar: cerebral blood flow in the patients without dizziness. Gray bar: cerebral blood flow in the patients with dizziness. Significant difference between without dizziness and with dizziness (*p < 0.05, **p < 0.01)
appeared in [5] and [6].
E: Comparison of the cerebral blood flow between the patients with pre-treatment and post-treatment of Ibudilast at rest. Blank bar: cerebral blood flow in the patients with pre-treatment of Ibudilast. Gray bar: cerebral blood flow in the patients with post-treatment of Ibudilast. Significant difference between without dizziness and with dizziness (*p < 0.05) appeared only in [6].
F: Comparison of the cerebral blood flow between the patients with pre-treatment and post-treatment of Ibudilast after acetazolamide loading. Blank bar: cerebral blood flow in the patients with pre-treatment of Ibudilast. Gray bar: cerebral blood flow in the patients with post-treatment of Ibudilast. Significant difference between without dizziness and with dizziness (*p < 0.05) appeared in [5],[6],[7],and [8].
The actual number of patients who suffered dizziness due to stroke is high unexpectedly. Dizziness after stroke has been considered as a result of vertebro-basilar insufficiency due to low perfusion of the brainstem and cerebellar hemisphere in generally. The pathophysiology of the relationship between stroke and dizziness has not been clarified yet, especially in concerned with the cerebral cortical origin. We found that in stroke patients with dizziness, a significant decrease in the CBF was found in both PIVC and VISC compared with patients without dizziness. From this evidence, both cortices of PIVC and VISC where closely related to control of the dizziness as described in many previous studies make us reconfirm that these cortices have major role also in the patients of stroke with dizziness. After loading of ACZ, vasomotor-reactivity in the areas of the VISC had not recovered while it was recovered in the PIVC even in the patients with dizziness. From these evidences, the reciprocal inhibition from the PIVC to the VISC might be dominant in the visual vestibular interaction in the stroke patients with dizziness. In patients treated with ibdilast, the amelioration of dizziness was accompanied by a significant increase in the CBF in only the VISC. Furthermore, we found that post-loading of ACZ in patients treated with ibdilast, the CBF was significantly increased in both the VISC and PIVC where ameliorated the vasomotor reactivity. These findings showed the dizziness after stroke is able to be reversible through ameliorate the functionally disrupted loop between visual-vestibular cortices where low perfusion of VISC and PIVC. The temporally cortical dysfunction causing dizziness after stroke can be recovered using some medicine such as ibdilast. To prove the post-stroke dizziness, the functional brain imaging such as PET, SPECT and XeCT might be useful by measuring the cortical low perfusion of the brain.
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grants-in-aid for Scientific Research no. 26430054 and 16k10788), and Japan Agency for Medical Research and Development (AMED; No.16ek0109182h0001).
Ethics statement
This study was carried out in accordance with the recommendations of Kumamoto Neurosurgical Hospital Institutional Review Board with written informed consent from all subjects. The protocol was approved by the Institutional Review Board.
Authorship contributions
NI contributed to the conception and design of the study; NI and SG contributed to data collection and analysis; NI and SG contributed to writing and revising the manuscript.
- Leigh RJ, Zee DS. The Neurology of Eye Movements. New York: Oxford University Press 1999.
- Baloh RW. The dizzy patient. Postgrad Med. 1999; 105: 161-164. PubMed: https://pubmed.ncbi.nlm.nih.gov/10026710/
- Tusa RJ. Dizziness. Med Clin North Amer. 2003; 87: 609-641. PubMed: https://pubmed.ncbi.nlm.nih.gov/12812406/
- Brandt T, Strupp M. General vestibular testing. Clin Neurophysiol. 2005; 116: 406-426. PubMed: https://pubmed.ncbi.nlm.nih.gov/15661119/
- Burt CW, Schapper SM. Ambulatory care visits to physician offices, hospital out-patient departments, and emergency departments. United States, 1999-2000. Vital Health Stats. 2004; 13: 161-714.
- Kerber KA, Brown D, Lisabeth, LD, Smith MA, Morgenstern JB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department. A population-based study. Stroke. 2006; 37: 2484-2487. PubMed: https://pubmed.ncbi.nlm.nih.gov/16946161/
- Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. 1954.
- Grusser OJ, Pause M, Schreiter U. Neuronal response in the parieto-insular vestibular cortex of alert Java monkeys (Macaca fascicularis). (1990) In: Physiological and Pathological Aspects of Eye Movements. 251-270.
- Gruccer OJ, Pause M, Schreuter U. Localization and responses of neurons in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis). J Physiol. 1990; 430: 537-557. PubMed: https://pubmed.ncbi.nlm.nih.gov/2086773/
- Guldin W, Grusser OJ. The anatomy of the vestibular cortices of primates. In: M. Collard, M. Jeannerord, Y. Christen (eds) Le cortex vestibulaire, Ipsen, Boulogne. 1996; 17-26.
- Wenzel R, Bartenstein P, Dieterich M, Danek A, Weindl A, et al. Deactivation of human visual cortex during involuntary ocular oscillations: A PET activation study. Brain. 1996; 119: 101-110. PubMed: https://pubmed.ncbi.nlm.nih.gov/8624674/
- Tiecks FP, Planck J, Harberl RL, Brandt T. Reduction in posterior cerebral artery blood flow velocity during caloric vestibular stimulation. J Cerebr Blood Flow Metab. 1996; 6: 1379-1382. PubMed: https://pubmed.ncbi.nlm.nih.gov/8898715/
- Brandt T, Bucher SF, Seelos KC, Dieterich M. Reciprocal inhibitory visual-vestibular interaction: Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain. 1998; 121: 1749-1758. PubMed: https://pubmed.ncbi.nlm.nih.gov/9762962/
- Ombergen AV, Heine L, Jillings S, Roberts RE, Jeurissen B, et al. Altered functional brain connectivity in patients with visually induced dizziness. NeuroImage: Clinical. 2017; 14: 538-545. PubMed: https://pubmed.ncbi.nlm.nih.gov/28331800/
- Inoue N, Fuyuta S. Cerebral blood flow in the visual and parieto-insular vestibular cortices in the patients after cerebral ischemia with or without dizziness. Int J Neuro Disord Interv. 2015; 1: 101-104.
- Inoue N, Harada M. Effect of ibudilast on non-specific symptoms in patients with chronic cerebral ischemia. Artzneimittelforschung. 2008; 58: 277-282. PubMed: https://pubmed.ncbi.nlm.nih.gov/18677969/
- Inoue N, Fukuda S. Inada T. Sameshima E., Tokushima Y et al. Effect of ibudilast on the reciprocal inhibitory visual-vestibular interaction closely related to dizziness after cerebral ischemia. J Stroke Cerebrovasc Dis. 2014; 23: 51-55. PubMed: https://pubmed.ncbi.nlm.nih.gov/23085301/
- Deutschlander A, Benses S, Stephan T, Schwaiger M, Brandt T et al. Sensory system interaction during simultaneous vestibular and visual stimulation in PET. Hum Brain Mapp. 2002; 16: 92-103. PubMed: https://pubmed.ncbi.nlm.nih.gov/11954059/
- Naito Y, Tateya I, Hirano S, Inoue M, Funabiki K, et al. Cortical correlates of vestibulo-ocular reflex modulation: A PET study. Brain. 2003; 126: 1562-1578. PubMed: https://pubmed.ncbi.nlm.nih.gov/12805122/
- Dieterich M, Bauerman T, Best C, Stoeter P, Schlindwein P. Evidence for cortical visual substitution of chronic bilateral vestibular failure (an fMRI study) Brain. 2007; 130: 2108-2116. PubMed: https://pubmed.ncbi.nlm.nih.gov/17575279/