More Information
Submitted: November 19, 2020 | Approved: December 15, 2020 | Published: December 15, 2020
How to cite this article: Riccioni L, Cremonini AM, Gessaroli M. Primary intracranial Hodgkin’s lymphoma after a blunt trauma: A case report. J Neurosci Neurol Disord. 2020; 4: 079-083.
DOI: 10.29328/journal.jnnd.1001039
ORCiD: orcid.org/0000-0002-3333-1717
Copyright License: © 2020 Riccioni L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hodgkin’s lymphoma; Intracranial; Central nervous system; Trauma; EBV
Primary intracranial Hodgkin’s lymphoma after a blunt trauma: A case report
Luca Riccioni1*, Anna Maria Cremonini2 and Manlio Gessaroli3
1O.U. of Anatomic Pathology, M. Bufalini Hospital, Cesena, Italy
2O.U. of Neurosurgery, M. Bufalini Hospital, Cesena, Italy
3O.U. of Maxillofacial Surgery, M. Bufalini Hospital, Cesena, Italy
*Address for Correspondence: Luca Riccioni, O.U. of Anatomic Pathology, M. Bufalini Hospital, Cesena, Italy, Tel: ++39 3478856268; Email: [email protected]; [email protected]
We report a case of 30-year-old immunocompetent man, with a previous history of cranial-facial trauma, who presented with progressive left exophthalmos due to an intracranial left frontal-ethmoidal-orbital mass. Histology of the resected tumor revealed a classical Hodgkin’s Lymphoma (HL). Epstein-Barr virus encoded RNA/EBER was detected in typical Hodgkin and Reed-Sternberg cells. After postoperative radiotherapy and chemotherapy administration, the patient remains free of systemic disease or recurrence on 4 years of follow-up.
Intracranial involvement by HL has rarely been described, mostly as a late localization or as a recurrence of a disseminated disease, in a setting of immunosuppression. Primary HL of the central nervous system occurring as an isolated disease is even more uncommon, with only 16 reported cases documented to date. The prognosis of these rare cases appears comforting with appropriate treatment. Tumor resection and, in appropriate cases, treatment with radiation and/or chemotherapy seem to warrant a durable response. For this reason a systemic disease should be excluded in all cases intracranial HL by a comprehensive work-up.
To the best of our knowledge, this case represents the first report that documents the association of intracranial HL and local trauma with subsequent intracranial infection.
Primary central nervous system lymphomas (PCNSL) account for 2.7% of the malignant disease of the central nervous system (CNS) [1]. Most of cases are intracerebral aggressive diffuse large B cell lymphoma, or indolent marginal zone with a meningeal dural localization. Intracranial involvement by Hodgkin’s lymphoma (HL) is rarely encountered, more frequently in the setting of immunosuppression. It occurs in 0.2% - 0.5% of patient with HL [2] and the majority and these cases had typical systemic manifestation of Hodgkin’s disease (HD) prior to the development of cerebral and intracranial localizations.
Herein we describe a new case of primary intracranial HL that occurred after a serious blunt facial trauma, with no evidence of systemic disease. To our knowledge this is the first report that documents this association. A review the pertaining literature to HL and CNS is provided.
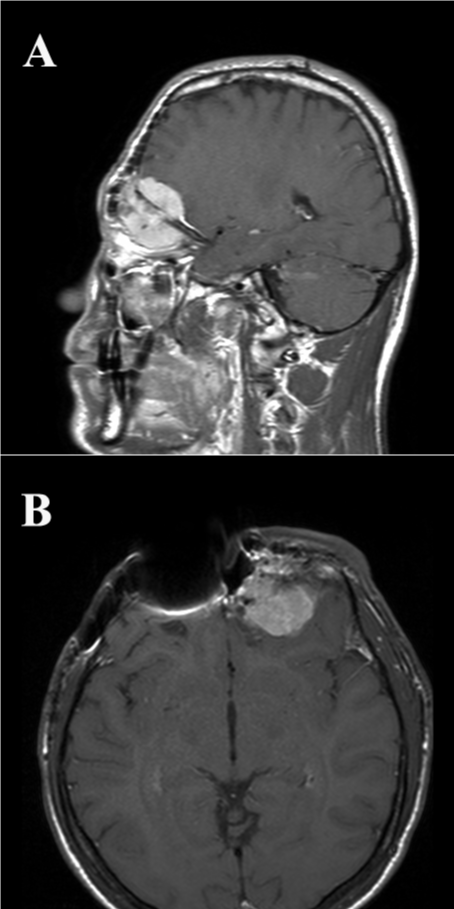
A 30-year-old immunocompetent male presented to Hospital with a progressive left esophtalm. He had a serious cranial-facial trauma six years before, which was treated with surgical intervention twice, firstly, after two years, for reconstruction of his skull base and another year after, for treatment of a local intracranial infection. Magnetic resonance imaging (MRI) scans showed an intracranial left frontal-ethmoidal-orbital mass, with contrast enhancement on T1 weighted images (Figure 1). Two months later he underwent craniotomy and the lesion was totally resected.
Figure 1: Sagittal (A) and transverse (B) T1 MRI images post-gadolinium injection demonstrate a left-sided enhancing lesion infiltrating the ethmoidal-orbital region and the cerebral frontal lobe. Artifacts due to protesic material are present.
The specimen was routinely processed for histology. Sections were stained with Haematoxylin and Eosin (&E). Immunohistochemical and in situ hybridization (ISH) studies were respectively performed with pre-diluted commercially available antibodies and EBV-encoded mRNA/ EBER probe, all from Novocastra, using Leica Bond Max automated autostainer. The reaction product was detected with diaminobenzidine chromogen.
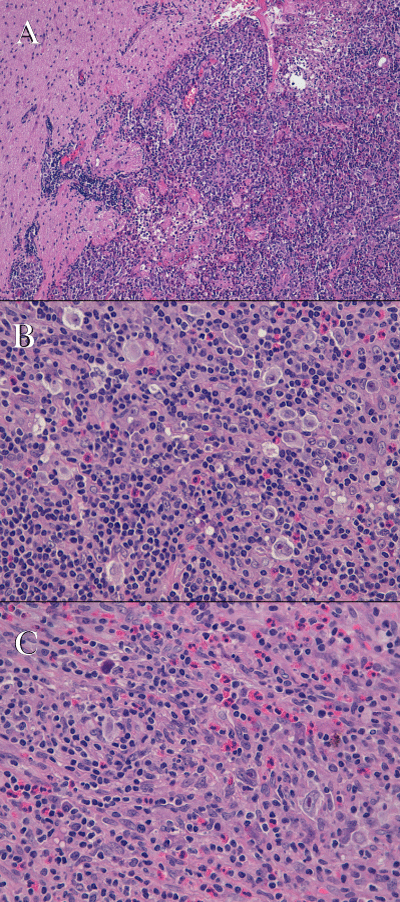
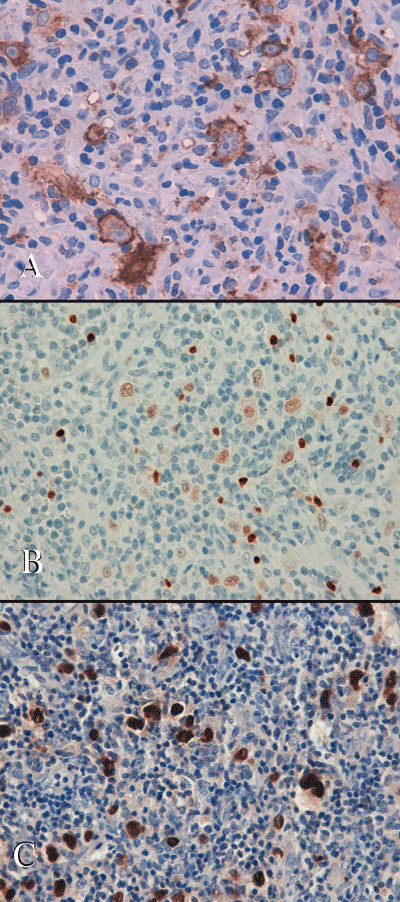
Histologically the tumor consisted of fragments of lymphoid tissue infiltrating the brain parenchyma at the periphery and focally covered by mucosa of ethmoidal sinuses. It was characterized by the presence of numerous large sized mononuclear Hodgkin’s cells, with vesicular nuclei and prominent eosinophilic nucleoli. There were also rare bi-nucleated Reed-Sternberg cells and tumor cells that exhibited a “lacunar” morphology with perinuclear halo. Tumor cells were scattered, either within a lymphocyte depleted fibrillar background, admixed with fibroblasts, small lymphocytes, plasma cells and numerous eosinophils, either within a lymphocytic-rich inflammatory areas close to the cerebral tissue (Figure 2). Immunohistochemistry demonstrated that tumor cells with Hodgkin’s morphology were diffusely positive for CD30, IRF4/MUM-1 and HLA-DR, focally immunoreactive for CD15 (paranuclear, dot-like positivity). They showed a faint nuclear positivity for PAX-5 and were negative for CD45-LCA, ALK protein, CD20, CD5, CD2, CD8, CD56, CD57, Granzyme-B, GFAP and cytokeratin AE1/AE3. The small reactive lymphocytes showed a prevalent T phenotype, without any expression of cytotoxic markers. There was no expression of CD21, S100 and CD1a. The Hodgkin’s cells resulted diffusely positive for Epstein Barr virus (EBV)-encoded latent membrane protein-1 (LMP-1); ISH for EBV (EBER) resulted positive (Figure 3). The overall results were consistent with a classical HL, with features of the lymphocyte depleted subtype.
Figure 2: Tumor resected from the brain: A) the image display the jagged boundary between the brain parenchyma and lymphomatous infiltrate (H&E; original magnification 2.5x); B) numerous Hodgkin’s and lacunar cells are readily seen admixed with small lymphocytes and eosinophils (H&E; original magnification 40x); C) lymphocyte’s depleted areas with Hodgkin’s and binucleated Reed Sternberg cells (H&E; original magnification 40x).
Figure 3: Immunohistochemical stains: A) Hodgkin’s cells are highlighted by CD30 antibody; B) Hodgkin’s cells show a faint nuclear positivity with Pax-5 antibody, compared with the intense immunoreactivity of the scattered small B lymphocytes. C) ISH: neoplastic cells show an intense positivity for EBV (EBER).
The post-surgical recovery was uneventful. The computed tomography (CT) assisted and pathologic staging with bone marrow examination, performed after the HL diagnosis, gave all negative results. The patient was subsequently treated with four cycles of chemotherapy with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), followed by 30.6 Gy radiotherapy to the left orbital region. The patient is alive and disease free at 11 years of follow-up, with no systemic manifestations of HL, neither local recurrences of the disease.
A case of primary intracranial HL has been described in an immunocompetent male with a past history of facial trauma and subsequent local infection. The patient had no signs of recurrence or systemic disease on follow-up for four years. Because of the consequences of the trauma to the skull base and the intracranial extension of the tumor at time of it’s discovering from the ethmoidal-orbital regions to the left frontal lobe, the site of origin cannot be restricted to one of these three different, but contiguous, anatomic locations.
Rare cases of intraorbital HL, with skull base and dural infiltration, and of nasopharyngeal HL with intracranial extension, have been described in patient with systemic Hodgkin’s disease [3-5].
Primary intracranial HL at presentation is an exceedingly rare occurrence. Since the first case report of Ashby, et al. in 1988 [6], convincingly supported by a limited immunohistochemical assay, additional 30 cases of initial presentation of intracranial HL have been reported to date, as isolated case reports and more recently in two case series [2,5,7-21]. Among these cases, only 16 were an isolated intracranial finding, with no evidence of a systemic disease [2,5-11,17-21], neither at staging, nor at follow-up (Table 1). Additional 16 cases of secondary involvement of the brain or spine by HL have been described, mainly in patients with a history of autoimmune disease or immunosuppression, which may be related to HIV infection [5,22-27]. Two of these cases were an intracranial recurrence of HL in HIV-positive male patients, with evidence of EBV infected tumor cells [23,24]. Another more recent case was a 65-year old man who developed isolated intracerebral EBV-positive HL, almost 8 years after the diagnosis of chronic lymphocytic leukemia/small lymphocytic lymphoma. Authors advocated a potential role to reactivate a latent EBV infection to the immunosuppression-related effects of fludarabine-based combination therapies for CLL [15]. In immunosuppressed patients PCNSL, virtually always arises from EBV-infected lymphocytes. Conversely the pathogenesis in immunocompetent patients is not so well-documented. We can speculate that the trauma was a predisposing factor for the development of the lymphoma in the case described here. Traumas may be responsible of prolonged or repeated inflammation of the tissue, or create a locus of minoris resistentiae, favoring viral infections, such as Epstein-Barr virus, or may attract peripheral blood lymphoid cells that are stimulated to proliferate locally and/or to undergo clonal selection and thus progress to a monoclonal neoplastic state. To our knowledge, five previous case reports have described the development of malignant lymphoma at site of a previous trauma in the head and neck and ocular region [28], but none of these cases was a HL. The clinical history of the patient led clinicians and pathologist to consider the differential diagnosis mainly with an inflammatory pseudotumor arising from the orbital-ethmoidal region or with a space occupying lesion due to an infectious etiology. The presence of numerous mononuclear Hodgkin’s cell with the proper immunophenotype, allowed the differential diagnosis with a pseudotumor and other histotypes of PCNS.
| Table 1: Cases of primary intracranial HL with no evidence of systemic disease at staging and follow-up. | ||||||
| Case | Year, author | age/sex | Disease site | Type HL | Treatment | Outcome at last FU |
| 1 | 1988, Ashby | 62/M | Fronto-parietal/meningeal R | NS | RT 4000 cGy; IT CT | NED (14 m) |
| 2 | 1990, Sickler | 84/F | Parieto-occipital/ intraparenchymal R | NOS | RT 3500 cGy; | NED 8 m |
| 3 | 1992, Clark | 53/F | Cerebellar R | NS | RT 4500 cGy | NED 6 m |
| 4 | 1999, Klein | 54/M | Occipital/subcortical R | NS | RT 3600 cGy WB boost 1400 cGy +CT | NED 16 m |
| 5 | 2000, Johnson | 55/F | Cerebellar tentorium | NS | RT operative site 14 Gy; WBRT 36 Gy | NED 8 m |
| 6 | 2000, Biagi | 52/M | Temporo-parietal L | NS | RT 300 cGy WB boost 500 cGy | NED 21 m |
| 7 | 2000, Herrlinger | 66/F | Fronto-parietal/cortical L | MC | 45 Gy WBRT, 2 x PCV CT | CR 18 m |
| 8 | 2008, Gerstner | 58/F | NA | NOS | WBRT (35 Gy) | CR 90.3 m |
| 9 | “ “ | 60/F | NA | NS | WBRT | NED 1 m |
| 10 | 2011, Foo | 58/M | Temporal L | NOS | WBRT (30 Gy) | RD, 14 m |
| 11 | 2012, Gessi | 77/M | Cerebellar L | MC | RT (38 Gy) | NED 8 m |
| 12 | “ “ | 59/M | Brainstem L | MC | NA | NA |
| 13 | 2013, Kresak J | 70/M | Cerebellar L | NS | RT | NED 10 y |
| 14 | “ “ | 72/F | Cerebellar dural based | NS | RT | NED 6 m |
| 15 | 2014, Sharaf | 77/M | Cerebellar L | MC | RT | NED 7 m |
| 16 | 2019, Alfaseh | 38/M | Intra-axial IV ventricle | MC | WBRT + CT (ABVD) | NED 7 y |
| 17 | present case | 30/M | Frontal - orbital - ethmoidal L | LD | ABVD CT, 4 cycles; RT 30.6 Gy left orbital region |
NED 30 m |
| L: Left; R: Right; F: Female; M: Male; NA; Not Available; NS: Nodular Sclerosis; MC: Mixed Cellularity; LD: Lymphocyte Depleted; NOS: Not Otherwise Specified; CT: Chemotherapy; IT: Intrathecal; RT: Radiotherapy; WBRT: Whole Brain Radiotherapy; NED: No Evidence of Disease; CR: Complete Remission; RD: Recurrent Disease (Intracranial); M: Months; Y: Years. | ||||||
Nodular sclerosis and mixed cellularity are the more frequently reported variants of CNS-HL [29]. However a recent larger series did found no association with a particular HL histology. Moreover is difficult to sub classify this disease in limited diagnostic biopsy, where the WHO sub classification criteria are not clearly reproducible.
Intracranial HL involves the brain either as a mass or as a diffuse infiltrate of the meninges. Intraparenchymal lesions are more frequent, probably due to not contiguous hematogenic spread of the disease. Dural involvement mimics radiologically meningioma as well as an inflammatory mass and seems to be much more common than parenchymal lesions in advanced stages of HD [9,13-15,22,25]. So when a dura-based lesion with imaging consistent with meningioma is discovered in a patient affected by HL, the possibility of an intracranial localization of the disease should be always considered.
There is no a standard therapeutic approach to CNS-HL, which usually varied with stage and presentation of the disease. Cases diagnosed concomitantly or at relapse of a widespread HL, usually received a combination of radiation and chemotherapy. At difference, patients with isolated primary intracranial HD more frequently underwent surgical tumor resection and received partial or whole brain radiotherapy alone.
Patients with IC-HL cerebral disease discovered at presentation seem to fare well, more than patients with cerebral disease at relapse [29]. The majority of cases of primary CNS-HL and systemic HD with primary CNS involvement, achieved complete remission of the disease and only 2 out of the 30 cases reported in literature died of systemic or cerebral progressive disease [2,5,7-18]. Conversely in AIDS and immunosuppressed patients with CNS-HL localizations the prognosis is still dismal, with shorter response to treatment, frequent relapses and dead of disease.
In conclusion, even if no long term follow-up data are available, isolated intracranial presentations of HL seem to have a favorable prognosis after surgical removal and adjuvant radiotherapy and chemotherapy administration.
- Bhagavathi S, Wilson JD. Primary central nervous system lymphoma. Arch Pathol Lab Med. 2008; 132: 1830-1834. PubMed: https://pubmed.ncbi.nlm.nih.gov/18976024/
- Klein R, Mullges W, Bendszus M, Woydt M, Kreipe H, et al. Primary intracerebral Hodgkin’s disease: report of a case with Epstein-Barr virus association and review of the literature. Am J Surg Pathol. 1999; 23: 477-481. PubMed: https://pubmed.ncbi.nlm.nih.gov/10199479/
- Sahjapaul, Elisevich K, Allen L. Hodgkin’s disease of the orbit with intracranial extension. Ophthalmic Surg Lasers. 1996; 27: 239-242. PubMed: https://pubmed.ncbi.nlm.nih.gov/8833130/
- Mallouh AA. Nasopharyngeal Hodgkin’s disease with intracranial extension in a child. Med Pediatr Oncol. 1989; 17: 174-177. PubMed: https://pubmed.ncbi.nlm.nih.gov/2704339/
- Gerstner ER, Abrey LE, Schiff D, Ferreri AJM, Lister A, et al. CNS Hodgkin lymphoma. Blood. 2008; 112: 1658-1661. PubMed: https://pubmed.ncbi.nlm.nih.gov/18591379/
- Ashby MA, Barber PC, Holmes AE, Freer CE, Collins RD. Primary intracranial Hodgkin’ disease. Am J Surg Pathol. 1988; 12: 294-299. PubMed: https://pubmed.ncbi.nlm.nih.gov/3354756/
- Sickler G, Hanson S, Hsu S, Papasozomenos SC. Primary intracerebral Hodgkin's disease: a case report. Clin Neuropathol. 1990; 9: 143-147. PubMed: https://pubmed.ncbi.nlm.nih.gov/2364594/
- Clark W, Callihan T, Schwarting R, Fontanesi J. Primary intracranial Hodgkin's lymphoma without dural attachment: case report. J Neurosurg. 1992; 76: 692-695. PubMed: https://pubmed.ncbi.nlm.nih.gov/1545264/
- Johnson M, Kinney M, Scheithauer B, Briley RJ, Hamilton K, et al. Primary intracerebral Hodgkin's disease mimicking meningioma: case report. Neurosurgery. 2000; 47: 454-457. PubMed: https://pubmed.ncbi.nlm.nih.gov/10942021/
- Biagi J, MacKenzie R, Lim M, Sapp M, Berinstein N. Primary Hodgkin's disease of the CNS in an immunocompetent patient: a case study and review of the literature. Neuro-oncology. 2000; 2: 239-243. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1920596/
- Herrlinger U, Kllingel K, Meyermann R, Kandolf R, Kaiserling E, et al. Central Nervous system Hodgkin’s lymphoma without systemic manifestation: case report and review of the literature. Acta Neuropathol. 2000; 99: 709-714. PubMed: https://pubmed.ncbi.nlm.nih.gov/10867808/
- Deckert-Schluter M, Marek J, Setlik M, Markova J, Pakos E, et al. Primary manifestation of Hodgkin’s disease in the central nervous system. Virchows Archives. 1998; 432: 477-481.
- Figueroa B, Brown J. Hodgkin's lymphoma of the CNS masquerading as meningioma. J Clin Oncol. 2004; 22: 4228-4230.
- Apollonsky N, Morris E, Johnson A, Bhuiya T, Karayalcin G. Intracerebral presentation of Hodgkin disease mimicking meningioma in a young woma. Case presentation with literature review. J Pediat Hematol Oncol. 2008; 30: 369-372. PubMed: https://pubmed.ncbi.nlm.nih.gov/18458571/
- Almhanna K, Wongchaowart N, Sweetenham J. Intracerebral Hodgkin’s lymphoma in a patient with chronic lymphocytic leukemia/small lymphocytic lymphoma: a case repport and literature review. Cancer Invest. 2009; 27: 215-220.
- Torgerson S, Olteanu H, Tinguely M, Fenske TS. Central nervous system Hodgkin lymphoma: case report and review of the literature. J Neurooncol. 2011; 102: 329-334. PubMed: https://pubmed.ncbi.nlm.nih.gov/20676729/
- Foo WC, Desjardins A, Cummings TJ. Primary intracerebral Hodgkin lymphoma with recurrence. Clin Neuropathol. 2011; 30: 75-79. PubMed: https://pubmed.ncbi.nlm.nih.gov/21329616/
- Gessi M, Kuchelmeister K, Kellner U, Ritter M, Morgner A, et al. Unusual clinico-pathological features in primary Hodgkin’s lymphoma of the central nervous system. Acta Neurochir. 2013; 155: 19-24. PubMed: https://pubmed.ncbi.nlm.nih.gov/23139103/
- Sharaf N, Lobo B, Lee J, Prayson RA. Primary Hodgkin lymphoma of the central nervous system. Case report/Journal of Clinical Neuroscience. 2014; 21: 1271-1273. PubMed: https://pubmed.ncbi.nlm.nih.gov/24589557/
- Alfaseh A, Rajeh MN, Hamed G. Primary central nervous system Hodgkin Lymphoma: A case discussion and a hypothesis on the etiology. Avicenna J Med. 2019; 9: 28-31. PubMed: https://pubmed.ncbi.nlm.nih.gov/30697523/
- Kresak JL, Nguyen J, Wong K, Davis R. Primary Hodgkin lymphoma of the central nervous system: two case reports and review of the literature. Neuropathology. 2013; 33: 658-662. PubMed: https://pubmed.ncbi.nlm.nih.gov/23530967/
- Montillo M, Scarpelli M, Discepoli G, Catarini M, Rychlicki F, et al. Meningioma-like cerebral relapse of Hodgkin’s disease. Leuk Lymphoma. 1994; 14: 185-187. PubMed: https://pubmed.ncbi.nlm.nih.gov/7920227/
- Nakayama H, Tokuuye K, Kagami Y, Sumi M, Murayama S, et al. Brain involvement in Hodgkin’s Disease: case reports and review of the literature. Radiation Med. 2000; 18: 205-208. PubMed: https://pubmed.ncbi.nlm.nih.gov/10972552/
- Morawa E, Ragam A, Sirota R, Nabhan C. Hodgkin’s Lymphoma Involving the CNS. J Clin Oncol. 2007; 25: 1437-1438. PubMed: https://pubmed.ncbi.nlm.nih.gov/17416864/
- Elwell VA, Carney L, Johns P, Grieve JP. Dural infiltration of metastatic Hodgkin’s lymphoma. Br J Neurosurg. 2008; 22: 439-440.
- Ramchandar K, Verhey LH, Jha NK, Murty NK, McMillan W. Intracranial Hodgkin’s lymphoma in an HIV positive patient. J Neurooncol. 2008; 89: 69-71. PubMed: https://pubmed.ncbi.nlm.nih.gov/18398570/
- Corti M, Fioti FV, Yampolsky C, Schtirbu R, Narbaitz M. Central nervous system involvement in Hodgkin’s lymphoma associated with Epstein –Barr Virus in a Patient with Aids: case report and review of the literature. Bra J Infect Dis. 2006; 10: 403-405. PubMed: https://pubmed.ncbi.nlm.nih.gov/17420914/
- Kriwalsky MS, Schroers R, Stricker I, Hollstein S, Kunkel M. Periorbital non-Hodgkin’s lymphoma after blunt trauma. Oral Surg Oral Med Oral Radiol Endod. 2010; 109: e56-e59. PubMed: https://pubmed.ncbi.nlm.nih.gov/20451833/
- Cheah CY, Brockelmann PJ, Chihara D, Moskowitz AJ, Engert A, et al. Clinical characteristics and outcome of patients with Hodgkin lymphoma with central nervous system involvement: An international multicenter collaboration. Am J Hematol. 2016; 91: 894-899. PubMed: https://pubmed.ncbi.nlm.nih.gov/27222367/