More Information
Submitted: April 06, 2021 | Approved: April 19, 2021 | Published: April 20, 2021
How to cite this article: Chiriboga N, Muñoz-Pareja J, Irazuzta J. Characterization of the immune response in neuroimmune disorders in children. J Neurosci Neurol Disord. 2021; 5: 022-025.
DOI: 10.29328/journal.jnnd.1001046
Copyright License: © 2021 do Chiriboga N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbrevations: NID: Neuroimmune Disorders; CNS: Central Nervous System; AIR: Adaptive Immune Response; Flow Cytometry
Characterization of the immune response in neuroimmune disorders in children
Nicolas Chiriboga1, Jennifer Muñoz-Pareja1 and Jose Irazuzta2*
1Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Florida College of Medicine, USA
2Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Florida College of Medicine-Jacksonville, USA
*Address for Correspondence: Jose Irazuzta, MD, Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Florida, College of Medicine-Jacksonville, 800 Prudential Drive. Jacksonville, Florida. 32207, USA, Tel: 904-202-8758; Email: [email protected]; [email protected]
Background: A misguided auto-reactive injury is responsible for several types of central nervous system (CNS) conditions in pediatrics. We propose that, in some of these conditions, the adaptive immune system has a common cellular immune pathogenesis, driven predominantly by T cells, despite variability on the phenotypical clinical presentation.
Methods: We have characterized the CD4+/CD8+ adaptive immune response (AIR) on pediatric patients presenting with clinical symptoms compatible with Neuroimmune Disorders (NID). Flow cytometry with deep immunophenotyping of T cells was performed on peripheral blood obtained during the acute clinical phase and compared to an age-matched cohort group (Co).
Results: We found that pediatric patients with confirmed NID, exhibit a pattern of dysregulation of CD4+ lineages associated with autoimmune processes.
Discussion: The autoimmune associated CD4+ dysregulation was associated with patients with NID, as compared to healthy controls and patients with non-autoimmune diagnoses. If we can improve our capacity for early accurate diagnosis and meaningful disease monitoring of pathogenic T cell subsets, we can both expedite disease detection and may serve as a guide to the administration of effective immunotherapeutic agents.
Neuroimmune disorders (NID) are a group of conditions in which the immune system is responsible for a misguided auto-reactive injury affecting the central nervous system (CNS) [1]. NID are common, for example, autoimmune encephalitis has become the most frequent form of encephalitis in developed countries [2]. They can be among the most challenging diseases to diagnose and treat, as their clinical symptoms are protean, and their diagnosis and treatment consist of invasive procedures requiring sedation in pediatric patients [3] and sometimes hazardous immune-suppression. NID have in common a cellular immune pathogenesis driven predominantly by T and B cells [1]. These disorders are the subject of intense scientific scrutiny as new pathogenic autoantibodies are being discovered at precipitous rates [4]. The evaluation of the efficacy of NID treatment attempts usually consists of a combination of clinical and radiological criteria that are subjective at best. Physicians and healthcare providers lack tools to establish a rapid diagnosis, or “metrics” that correlate with disease progression and/or heralds an efficacious therapeutic response [5].
The importance of developing a diagnostic tool that reveals the “state of the immune milieu” in pediatric NID, could be a significant support for clinical practice.
We sought to characterize the adaptive immune response of peripheral blood mononuclear cells (PBMC) of pediatric patients suspected of having NID. Utilizing flow cytometry, we focused on the deep immunophenotyping of CD4+ lineages associated with autoimmune processes.
Patient enrollment
All procedures were approved by local Institutional Review Boards and conducted in accordance with the Declaration of Helsinki. Prior to enrollment, informed consent was obtained from the patients’ legal guardians with ascent from the patients who were old enough and capacitated to provide this.
Patients aged from 2-18 years of age admitted or transferred to any of the participating pediatric institutions with suspicion of a NID or conditions that mimic its clinical presentation were eligible to participate.
Inclusion criteria: pediatric patients with rapidly progressive changes in consciousness or motor deficits, dyskinesia, or acute psychiatric disturbance.
Exclusion criteria: patients with known autoimmune disorders, CNS degenerative, vascular or infectious condition, with clinical effects from recreational drugs or adverse effects from therapeutic agents. Patients that may have received, prior to the acute clinical presentation, agents directed to modify the immunological response.
Conclusive diagnosis was reached utilizing CSF and serological results, MRI and or nerve conduction studies combined with medical trajectory and opinions from pediatric critical care, pediatric neurology, immunology and pediatric rheumatology consultants.
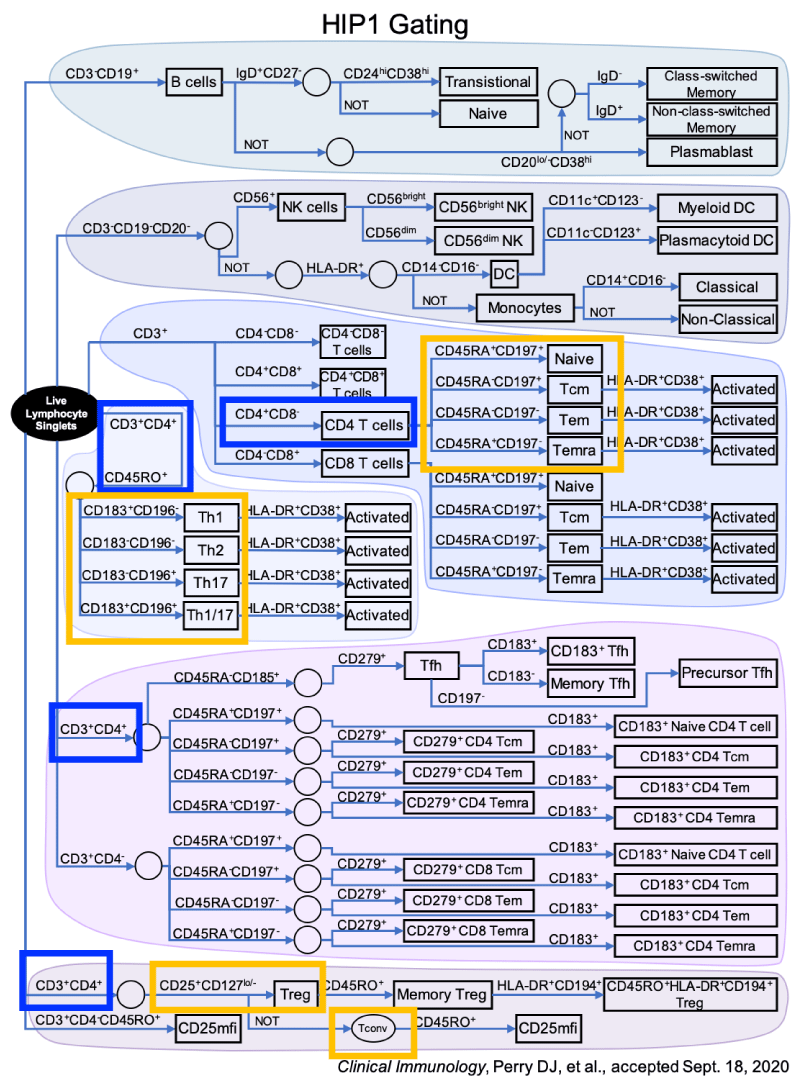
Blood was drawn upon clinical presentation. We performed a comprehensive flow cytometric immunophenotyping in PBMC of T cells with a focus on T effector (Teff) and T regulatory (Treg) cells. Direct immunofluorescence surface staining of whole blood samples with 6 antibody panels was performed. Gating was performed to evaluate for CD3- (CD19+ and CD19-) cells, CD3+ CD4+ (CD45+, CD45-, and CD25+) cells. Details for gating can be found on figure 1.
Figure 1: Gating utilized for flow cytometry. This figure describes cell gating performed for the flow cytometric analysis for this study. This was based on findings on studies by Perry et.al based on other auto-immune conditions. This panel allows for multiple cell lines to be studied with emphasis on Teff and Treg cells.
After the data was collected and subsequent to hospital discharge, patients were allocated into groups by final diagnosis: Group 1: confirmed NID, Group 2: control, a cohort group of age-matched controls constructed from an internal repository, Group 3: confirmed non-NID enrolled patients. Patients with an inconclusive diagnosis are described but do not participate in further analysis.
Statistical analysis
Paired and Linear regression was utilized to find linear correlations between specific cell lines and diagnostic groups. We concentrated on cells in the CD4+ line and specific cellular lines characteristically associated with the regulatory autoimmune response. Statistical significance was established at 0.05.
Eighteen patients were enrolled, vital statistics and diagnosis are described in table 1. Conclusive diagnosis was attained between 3 days and 6 months post admission. The final diagnoses were 11 NID (specific NID subtypes in table 2), 2 viral etiologies, and 3 with a genetic etiology, 2 subjects had an inconclusive diagnosis, and were thus excluded from analysis.
| Table 1: Vital Statistics of Subjects. | ||
| Demographic | N = | Percentage |
| Age: | ||
| 02-May | 3 | 17% |
| 06-Oct | 7 | 39% |
| Nov-15 | 7 | 39% |
| ≥16 | 1 | 6% |
| Sex: | ||
| Male | 8 | 44% |
| Female | 10 | 66% |
| Table 2: Subjects’ NID diagnoses. | |
| Diagnosis | |
| NMDAr | 2 |
| Transverse myelitis | 4 |
| Guillain Barre | 2 |
| Miller Fisher variant | |
| NORSE | 1 |
Patients with NID shared an immune dysregulation that distinguished them from the age-matched cohort.
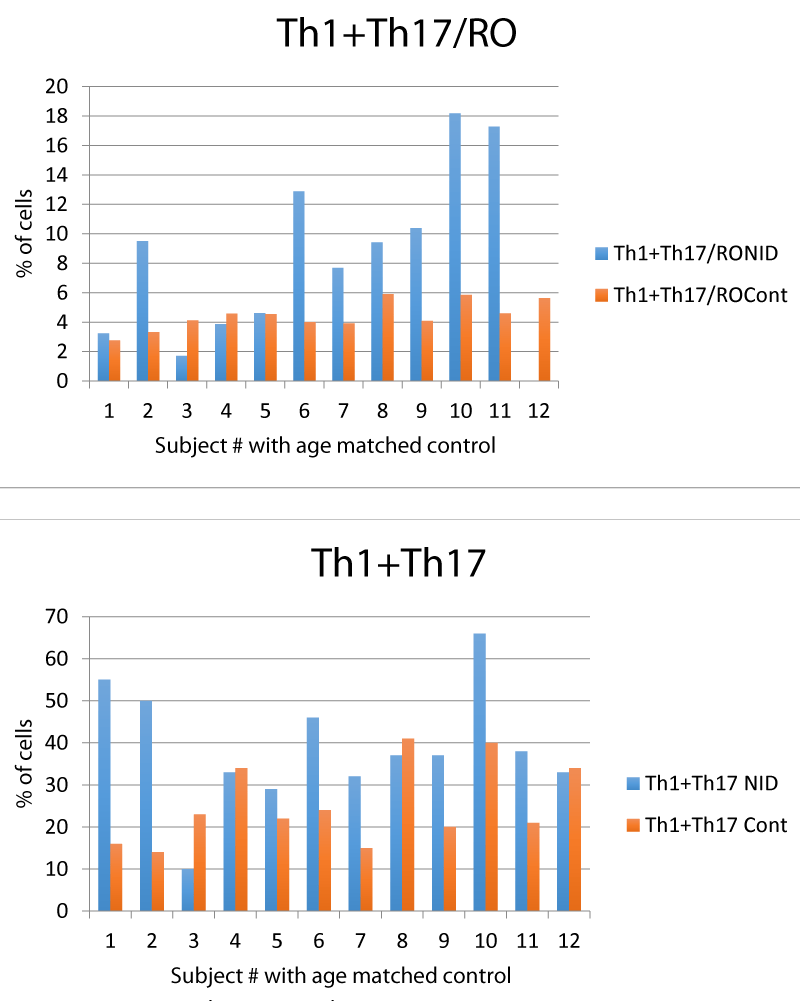
We found that in the NID group there was an expansion of the CD4+ lineage traditionally associated with autoimmune conditions of the Central Nervous System, Th1 and Th17 [6] (Table 3, figure 1). This clonal expansion was uncontrolled, as the ratio between autoreactive cells and regulatory cells in the CD4+ group (Th1+Th17/RO+ Treg), was skewed (Table 3, Figure 2).
| Table 3: CD4 cell lines by group. | ||
| Cell lineage | Mean/SD (in % of cells present) | p value |
| Th1 + Th17 | NID: 38.8 ± 14.2 | |
| Controls: 25.33 ± 9.5 | p = 0.02 | |
| Th1+Th17/RO+ Treg | NID: 10± 5.2 | |
| Controls: 4.9 ± 0.8 | p = 0.02 | |
| Non NID: 5.5 ± 3.4 | p = 0.05 | |
Figure 2: Gating utilized for flow cytometry.
In this pilot study, we found that pediatric patients with confirmed NID exhibit a pattern of dysregulation the adaptive immune response; more specifically, of lineages within the CD4+ complex hitherto associated with autoimmune conditions [6]. Multiple studies have shown that Th1 and Th17 cells, which the results of our study suggest are dysregulated in patients with NID, have a role in autoimmune processes of the CNS. Furthermore, there seems to be a failure of Treg cells to regulate the pro-inflammatory effect of Th1 and Th17 producing cells. This was also reproduced in our study [6].
Patients affected by NID, were found to possess a reduced number of controlling T regulatory cells in relation to the expansion of the T effector cell subsets associated with autoreactive cell lineage, Th1 + Th17 [6,7]. Moreover, there seem to be a failed attempt to produce more controlling Treg cells when we look at the behavior of the Tconv lineage that express CCR4. We interpret this finding as an inability to control a dysregulated self-injurious milieu [8].
A previous study by Lepennetier and Kowarik, performed in CSF, found that neuroinflammatory disorders correlate with immune deregulation and a shift in the quantity and quality of CD4, CD8 and NK cells [9]. This study excluded patients with antibody mediated encephalitis and it was done in adults. We focused on characterizing the adaptive immune response in the peripheral blood in children as we believe is a more accessible tool that can be serially repeated to understand the evolution of the disease after treatments attempts. There are serious constraints about analyzing CSF in children including the quantity of CSF needed (in the study by Kowarik 5-15 ml), the need for sedation, and the added difficulty and risk of lumbar punctures in children.
We cannot generalize for other non-NID conditions, as our data is scant with a significant age difference between groups 1 and 3.
The immune system does not act in randomness; it is rather an integrated network of cellular lines with tightly controlled feedback loops [10,11]. It is an autonomous system that, banning some underlying condition, exerts a servo-control mechanism capable of responding to non-self-antigens [12]. Serendipity (i.e. triggered by viral mimicry [13], underlying deficiency or a temporary lack of self-regulation [10] may initiate and perpetuate an autoimmune process. Discerning peripheral immune patterns that point to a de-coupling of the integrated network has the potential to be utilized as an “immune biomarker” of a CNS self-injurious pathobiology. Moreover, this servo-controlled system ultimately ought to “reset” to its normal state once efficacious therapies are employed [14], even though clinical signs of improvement may lag behind.
Our findings share some similarity to findings in autoimmune conditions affecting other organs(Asothai et al., 2015), including the CNS where the T effector cells Th1 and Th17 are strongly associated with self-reactivity [6,7,15,16]. By the same token, a reduction in number and function of regulatory (i.e. Treg) cells [17].
Uncovering Immune biomarkers for NID can have wide reaching implications. Immune cell lines don’t act on isolation but rather obey a complex choreography in reaction to different stimuli. This study is a first step in trying to characterize this reaction with aims to studying other cell lines. Previous studies have examined the use of algorithms based on cellular ratios to examine the coordination of the immune system in autoimmune conditions [18]. This coordination/auto-regulation is lost in autoimmune conditions [19]. Assessing these cellular lines in the context of their control feedback with an algorithm has the potential to be utilized as a clinical biomarker. A laboratory score from PBMC that is able to discriminate between autoimmune and other causes of CNS disorders, can be of great utility in the early identification of patients with NID. Moreover, if these assessments of interactions point to “weak links” in the regulatory mechanism it may provide an insight into potential therapeutic targets to restore homeostasis.
Limitations: translating our findings into biological insight possess challenges due to the limited number of observations. However, our pilot observations remain thought provoking and may serve as bases for future studies.
- Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, et al. Immunopathology of autoantibody-associated encephalitides: Clues for Pathogenesis. Brain. 2012; 135: 1622-1638. PubMed: https://pubmed.ncbi.nlm.nih.gov/22539258/
- Ramanathan S, Mohammad SS, Brilot F, Dale RC. Autoimmune encephalitis: Recent updates and emerging challenges. J Clin Neurosci. 2014; 21: 722–730. PubMed: https://pubmed.ncbi.nlm.nih.gov/24246947/
- Cellucci T, Van Mater H, Graus F, Muscal E, Gallentine W, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm. 2020; 7: e760. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7188473/
- Bigi S, Hladio M, Twilt M, Dalmau J, Benseler SM. The growing spectrum of antibody-associated inflammatory brain diseases in children. Neurol Neuroimmunol Neuroinflamm. 2015; 2: e92. PubMed: https://pubmed.ncbi.nlm.nih.gov/25909091/
- Neuteboom R, Wilbur C, Van Pelt D, Rodriguez M, Yeh A. The Spectrum of Inflammatory Acquired Demyelinating Syndromes in Children. Semin Pediatr Neurol. 2014; 24: 189–200. PubMed: https://pubmed.ncbi.nlm.nih.gov/29103426/
- Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of Autoimmunity. 2008; 31: 252–256. PubMed: https://pubmed.ncbi.nlm.nih.gov/18502610/
- Waite JC, Skokos D. Th17 response and inflammatory autoimmune diseases. Int J Inflamm . 2012. 2012:819467. PubMed: https://pubmed.ncbi.nlm.nih.gov/22229105/
- Asothai R, Anand V, Das D, Antil PS, Khandpur S, et al. Distinctive Treg associated CCR4-CCL22 expression profile with altered frequency of Th17/Treg cell in the immunopathogenesis of Pemphigus Vulgaris. Immunobiology. 2015; 220: 1129–1135. PubMed: https://pubmed.ncbi.nlm.nih.gov/26093920/
- Lepennetier G, Hracsko Z, Unger M, Van Griensven M, Grummel V, et al. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J Neuroinflamm. 2019; 16: 219.
- Korn T, Kallies A. T cell responses in the central nervous system. Nat Rev Immunol. 2017; 17: 179–194. PubMed: https://pubmed.ncbi.nlm.nih.gov/28138136/
- Stockinger B, Veldhoen M, Martin B. Th17 T cells: Linking innate and adaptive immunity. Semin Immunol. 2007; 19: 353–361. PubMed: https://pubmed.ncbi.nlm.nih.gov/18023589/
- Bar-Or A, Hintzen RQ, Dale RC, Rostasy K, Brück W, et al. Immunopathophysiology of pediatric CNS inflammatory demyelinating diseases. Neurology. 2016; 87: S12-129. PubMed: https://pubmed.ncbi.nlm.nih.gov/27572856/
- Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018; 17: 760–772. PubMed: https://pubmed.ncbi.nlm.nih.gov/30049614/
- Gastaldi M, Thouin A, Vincent A. Antibody-Mediated Autoimmune Encephalopathies and Immunotherapies. Neurotherapeutics. 2016; 13: 147-162. PubMed: https://pubmed.ncbi.nlm.nih.gov/26692392/
- Debnath M, Berk M. Th17 pathway-mediated immunopathogenesis of schizophrenia: Mechanisms and implications. Schizophr Bull. 2014; 40: 1412–1421. PubMed: https://pubmed.ncbi.nlm.nih.gov/24711545/
- Li S, Jin T, Zhang HL, Yu H, Meng F, et al. Circulating Th17, Th22, and Th1 cells are elevated in the guillain-barr?? syndrome and downregulated by IVIg treatments. Mediators of Inflammation. 2014.
- Prasad S, Hu S, Sheng WS, Singh A, Lokensgard JR. Tregs modulate lymphocyte proliferation, activation, and resident-memory T-cell accumulation within the brain during mcmv infection. PLoS ONE. 2015; 10: e0145457. PubMed: https://pubmed.ncbi.nlm.nih.gov/26720146/
- Guo H, Xun L, Zhang R, Gou X. Ratio of CD147high/CD147low in CD4+CD25+ T cells: A potential biomarker for early diagnosis and prediction of response to therapy for autoimmune diseases. Med Hypotheses. 2018; 115: 1–4. PubMed: https://pubmed.ncbi.nlm.nih.gov/29685186/
- Gastaldi M, Zardini E, Scaranzin S, Uccelli A, Andreetta F, Baggi F, et al. Autoantibody Diagnostics in Neuroimmunology: Experience From the 2018 Italian Neuroimmunology Association External Quality Assessment Program. Front Neurol. 2020; 10: 1385. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6971200/