More Information
Submitted: December 06, 2021 | Approved: December 30, 2021 | Published: January 03, 2022
How to cite this article: Hatim G, Chekrine T, Houjami M, Boughafour M, Bouchbika Z, et al. Pediatric brainstem glioma. J Neurosci Neurol Disord. 2022; 6: 001-004.
DOI: 10.29328/journal.jnnd.1001059
Copyright License: © 2022 Hatim G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Brainstem tumors; Pediatric glioma; Radiotherapy
Pediatric brainstem glioma
Ghita Hatim* , Tarik Chekrine, Majdouline Houjami, Mouna Boughafour, Zineb Bouchbika, Nadia Benchakroun, Hassan Jouhadi, Nezha Tawfiq, Abdelatif Benider and Souha Sahraoui
, Tarik Chekrine, Majdouline Houjami, Mouna Boughafour, Zineb Bouchbika, Nadia Benchakroun, Hassan Jouhadi, Nezha Tawfiq, Abdelatif Benider and Souha Sahraoui
Department of Radiation Oncology, Mohammed VI Center, Cancer Treatment, UHC Ibn Rochd, Casablanca, Morocco
*Address for Correspondence: Ghita Hatim, Faculty of Medecine and Pharmacy of Casablanca, Hassan II University, Mohammed VI Center, Cancer Treatment, UHC Ibn Rochd, Morocco, Email: [email protected]
Background and objectives: Brainstem gliomas are tumors of the central nervous system which have varying presentations and clinical courses. This study aims to analyze the frequency, clinical and therapeutic aspects of brainstem glioma.
Methods: We retrospectively analyzed the data from the record of the patients treated for brainstem glioma under the age of 20 between January 2007 and July 2020 in the Radiation Oncology department of the Ibn Rochd UHC.
Results: There were fifteen patients (10 males and 5 females). The mean age of onset was 12 years (range 8 - 14 years). The duration of symptoms varied from 1 month to 2 years. Nine of the patients had intracranial hypertension due to hydrocephalus, six had cranial nerve deficits at presentation, and five patients had cerebellar signs. The lesion was pontine in 12 cases. None of the patients had a tumoral resection, nine had a ventriculo-peritoneal shunt insertion for the hydrocephalus and three had a Stereotactic biopsy that revealed one astrocytoma grade 1, one low grade glioma and one glioblastoma. The radiotherapy was indicated in all the cases but only nine patients had a 3D radiotherapy with a total dose of 54 Gy. Three patients received chemotherapy. Six patients are still alive, two are lost to follow up and seven patients are dead with a mean survival period of 8 months.
Conclusion: Brainstem glioma is a devastating disease with a bad prognosis. The clinical presentation is variable and the management is multidisciplinary. Our study illustrates the importance of treatment by radiation.
Brainstem gliomas constitute 10% to 20% of children’s brain tumors [1,2] and less than 2% for adults [3]. This kind of tumor can be subdivided into diffuse pontine gliomas, tectal and cervicomedullary gliomas [4]. Pontine gliomas represent 80% of the brainstem gliomas [5]. They are usually infiltrative, have an aggressive course and are associated with a dismal prognosis [6]. Therapeutic options are limited, and brainstem gliomas are associated with high morbidity. The treatment is based on external radiation therapy, chemotherapy efficacy has not been proven and resection is still impossible in the diffuse infiltrative pontine lesions [7,8]. Generally associated with a poor prognosis, new imaging techniques demonstrated subsets with different evolutions and outcomes. In this work, we analyze our own experience with the management of brainstem’s pediatric tumors in the Radiation oncology department of the Ibn Rochd UHC. This study aims to analyze the frequency, clinical and therapeutic aspects of brainstem glioma.
A retrospective study of brainstem tumors treated in the Radiation oncology department of Ibn Rochd UHC between January 2007 and July 2020. Fifteen patients under the age of twenty were treated for brainstem tumors in all patients registered for a tumor of the central nervous system. The parameters studied were: age, circumstances of discovery, clinical and paraclinical aspects and the treatment performed. Patient information was obtained from the department’s registers and patient records.
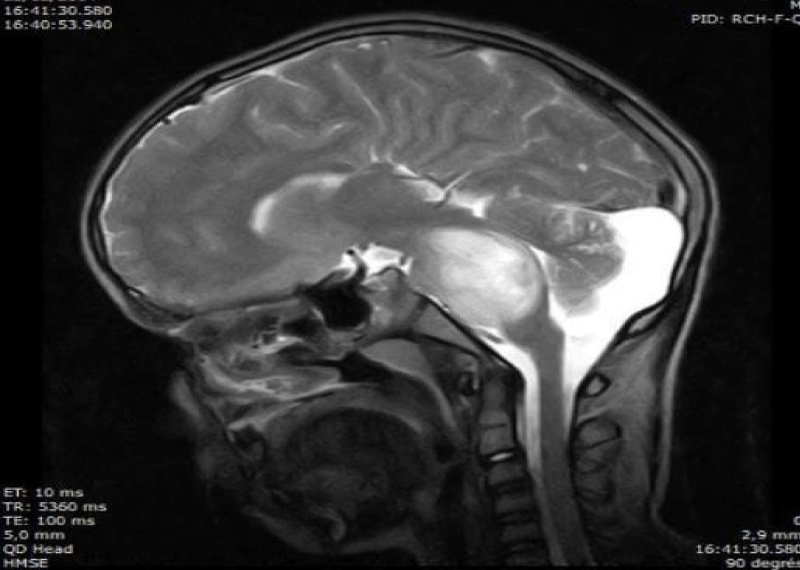
A total of fifteen patients diagnosed with a brainstem tumor were admitted during the study period, ten males and five females. The average age was 12 years with extremes ranging from 2 to 18 years of age; the diagnostic circumstances were the apparition of intracranial hypertension in nine cases (60%), cerebellar signs were seen in five cases (33%) and a Cranial nerve deficit revealing for one patient. Twelve patients (80%) had pontine lesions and the tumor was located in more than one location (midbrain and pons) three patients (20% of cases). Nine of the patients (60%) had intracranial hypertension due to hydrocephalus that was cured by a ventriculo-peritoneal shunt insertion. The diagnosis was confirmed by a stereotactic biopsy for three patients (20%) that revealed one astrocytoma grade 1, one low grade glioma and one glioblastoma, while the diagnosis was established by the typical appearance on brain MRI for twelve patients (80%) (Figure 1). None of the patients had a tumoral resection. Radiotherapy was indicated in all the cases but only nine patients underwent 3D radiation therapy, while two patients were lost to follow up and four died before they can access to their treatment by radiation. Total radiation dose varied from 50 to 54 Gy (Figures 2,3). Regarding radiotherapy side effects, all patients presented headaches and fatigue during the first week of treatment, two patients had nausea and vomiting and one patient presented confusion. Three patients received chemotherapy that combines Carboplatin to Vincristin with a good tolerance.
Figure 1: Cerebral MRI T2: pontic diffuse tumor (oncology department of the Ibn Rochd UHC Casablanca).
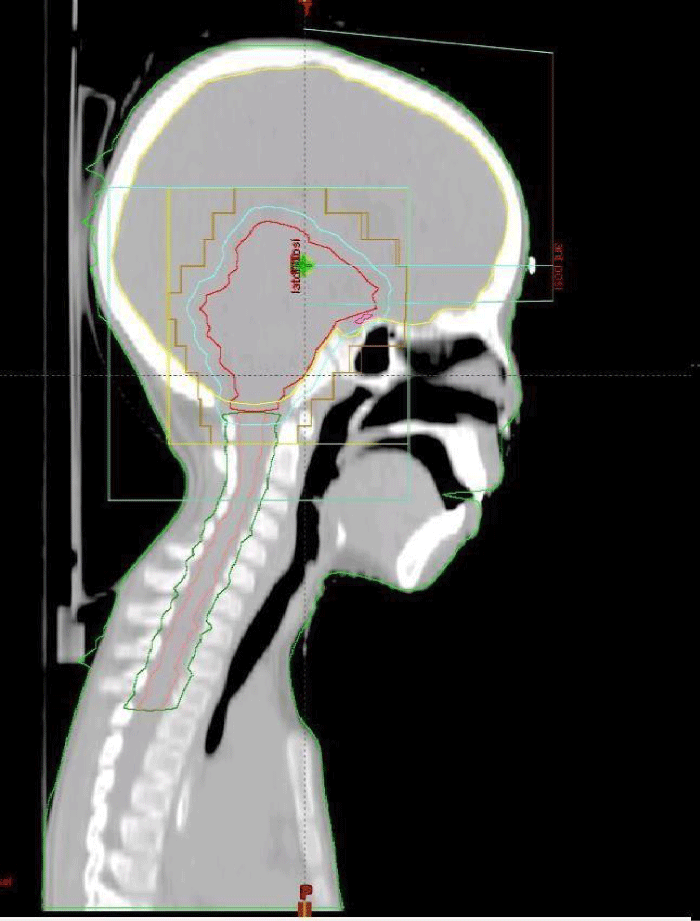
Figure 2: Target volume and dose distribution (Radiation oncology department of the Ibn Rochd UHC Casablanca).
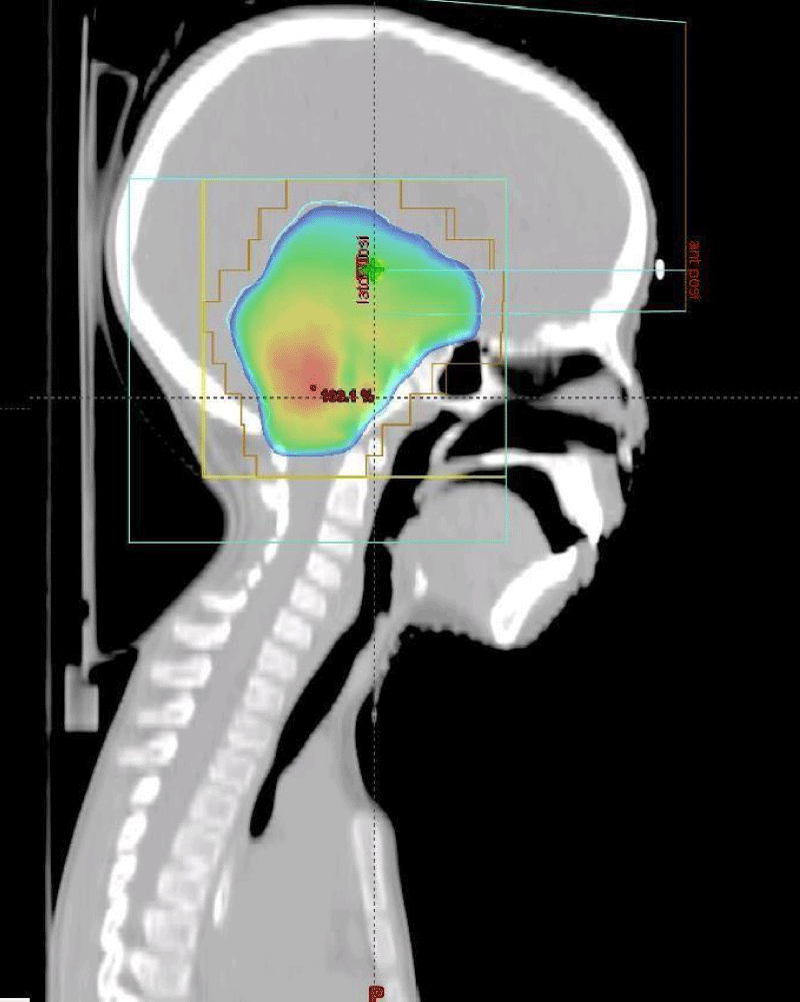
Figure 3: Target volume and dose distribution (Radiation oncology department of the Ibn Rochd UHC Casablanca).
Six patients (40%) are still alive, two are lost to follow up and seven patients are dead with a mean survival period of eight months with extremes ranging from five to fourteen months (Table 1).
| Table 1: Summary of patient observation. | ||||||
| Patients | Age | Sex | Symptoms | Treatment | RTH: Dose | Evolution |
| 1 | 2 years | Male | Motor pathways deficit Problem behaviors Insomnia |
Ventriculo- peritoneal shunt | - | Dead |
| 2 | 15 years | Male | Intracranial hypertension Cerebellar signs Cranial nerve deficits |
Ventriculo- peritoneal shunt Radiotherapy |
54 Gy | Dead |
| 3 | 11 years | Male | Motor pathways deficit | Radiotherapy | 54 Gy | Dead |
| 4 | 12 years | Male | Intracranial hypertension Vestibular disorders |
Ventriculo- peritoneal shunt | - | Dead |
| 5 | 18 years | Male | Cerebellar signs Cranial nerve deficits |
- | - | Lost to follow up |
| 6 | 19 years | Female | Intracranial hypertension Cerebellar signs Cranial nerve deficits |
Ventriculo- peritoneal shunt Radiotherapy |
54 Gy | Dead |
| 7 | 8 years | Male | Intracranial hypertension Cerebellar signs |
- | - | Dead |
| 8 | 10 years | Male | Cranial nerve deficits | Radiotherapy | 54 Gy | Alive (Supportive care) |
| 9 | 13 years | Female | Intracranial hypertension | - | - | Dead |
| 10 | 8 years | Female | Motor pathways deficit | Ventriculo- peritoneal shunt Radiotherapy |
54 Gy | Alive (Supportive care) |
| 11 | 19 years | Male | Intracranial hypertension Cranial nerve deficits |
Ventriculo- peritoneal shunt Radiotherapy Chemotherapy |
54 Gy | Alive (Supportive care) |
| 12 | 10 years | Male | Intracranial hypertension | Ventriculo- peritoneal shunt Radiotherapy |
50 Gy | Alive (Supportive care) |
| 13 | 7 years | Female | Intracranial hypertension Cranial nerve deficits |
Ventriculo- peritoneal shunt Radiotherapy Chemotherapy |
50 Gy | Alive (Supportive care) |
| 14 | 9 years | Male | Intracranial hypertension | Ventriculo- peritoneal shunt Radiotherapy Chemotherapy |
52 Gy | Alive (Supportive care) |
| 15 | 5 years | Female | Cerebellar signs Motor pathways deficit |
- | - | Lost to follow up |
Concerning patients who were treated by radiotherapy, three are dead and six are still alive with supportive care.
Pediatric Brainstem tumors represent 10% to 20% of the central nervous system tumors [4,9], referring to the Cancer Regional Register of Casablanca going from 2008 to 2012 [10], pediatric brain tumors represent 16.7% of all brain tumors. In our study, the average age was 12 years with extremes ranging from 2 to 19 years of age, making our results a little higher than the previous series [11,12]. In bibliographic references, there is no gender predilection [13] but according to recent and our results, we notice a little male predomination explained by the small number of studied patients. Intracranial hypertension syndrome dominated the clinical picture. Medical imaging has become invaluable in the diagnosis and management of brainstem gliomas; due to their location, getting a biopsy is almost impossible [14,15]. Conventional brain MRI is the master tool to get a diagnosis, it can help to distinguish between low grade and high grade brainstem tumors. They are in hyposignal T1 in 82% of cases, hypersignal T2, without any hemorragic, necrotic or cystic portion, and diffuse in 94% of cases [6,16].
The new central nervous system tumors classification «WHO 2016» is not only based on morphologic criteria, but also on some molecular parameters [17], it defines homogen groups in term of prognosis and the treatment response. In the fifth edition of the WHO Classification of Tumors of the Central Nervous System (WHO CNS5) recently published in 2021 which is an update of the 2016 WHO classification [18], the Diffuse Midline Gliomas H3 K27M-mutant has been recognized as a new diagnostic entity that should only include infiltrating gliomas arising in midline structures. It has also been revealed that H3 K27M mutation accelerates brainstem tumorigenesis of high grade gliomas from neonatal progenitor cells [19]. The diagnosis was confirmed by a stereotactic biopsy for 20% of the patients that revealed one astrocytoma grade 1, one low grade glioma and one glioblastoma, while the diagnosis was established by the typical appearance on brain MRI for 80% of the patients. The therapeutic strategy is based on the tumor’s histological aspects and its location. Big progress has been done in brainstem tumors surgical approach; they are no longer considered inoperable. In our study, none of the patients underwent tumoral resection surgery. For diffuse brainstem tumors, radiotherapy is still the unique treatment. Radiotherapy is the only treatment that has an impact on patient’s survival, it can control 50% to 70% of focal brainstem lesions [20-22]. For low grade focal brainstem glioma, the prescipted dose goes from 50.4 to 54 Gy/1.8-2 Gy per fraction, in some cases a dose of 45 Gy delivered in 25 fractions can be considered (children under the age of 5). For high grade diffuse brainstem glioma, the prescipted dose is 60 Gy/2 Gy per fraction. A recent study comparing Conventional fractionated radiotherapy to hypofractioned radiotherapy (39 Gy/3 Gy per fraction) has demonstrated that there is no impact on patient’s survival or radiation side effects [23].
Hypofractioned is recommended only because patients will pass less time on treatment with a better life quality [24]. Despite a lot of clinical trials about dose escalation or fractionation options, the conventional fractionated radiotherapy is still the best option. In our study, radiotherapy was indicated in all the cases but only 80% of the patients underwent a conventional fractioned 3D radiation therapy with a total dose of 54 Gy according to the international recommendations. The radiotherapy new technics, including the Intensity Modulated Radiotherapy (IMRT), the Volumetric-modulated arc therapy (VMAT), tomotherapy, protontherapy and the stereotactic radiotherapy aims to increase the dose received by the tumor with reducing radiation side effects.
Despite all the progress, the neuroprotection benefits are still not clear, and need more clinical trials [25]. Concerning protontherapy, literature data is in favor of neurocognitive, sensorial and endocrine benefits with protons compared to photons for all paediatric patients treated for brain tumours in a curative intend [26]. Greenberg demonstrated, in a study with 32 pediatric patients with low-grade gliomas of the brain or spinal cord were treated with proton RT the efficiency of protontherapy. The 6- year and 8-year rates of progression-free survival were 89.7% and 82.8%, respectively, with an 8-year overall survival of 100% [27]. The chemotherapy was indicated for only 3 of our patients. Longer survival in patients who were treated with intense chemotherapy following irradiation has been shown in a recent study [8], but the use of chemotherapy before or after radiation had been evaluated in many other studies with no clear benefits [7]. Carboplatin combined to Vincristin weekly is one of the most used drugs; this association has been compared to the «TPCV» combination that includes 6- thioguanine, procarbazine, CCNU and vincristine, in an american study for the treatement of low grade pediatric glioma; their efficiency was equal [28]. Meanwhile other drugs such as Bevacizumab [29] and Temozolomid [30] may be more indicated in case of progression after receiving a first line of chemotherapy or after irradiation.
Conclusion
Brainstem glioma is a devastating disease with a bad prognosis. The clinical presentation is variable and the management is multidisciplinary. Radiotherapy is still the unique treatment for diffuse brainstem tumors. Our study illustrates the importance of treatment by radiation.
- Farwell JR, Dohrmann GJ, Flannery JT. Central nervous system tumors in children. Cancer. 1977; 40: 3123–3132. https://pubmed.ncbi.nlm.nih.gov/201364/
- Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006; 24: 1266–1272. https://pubmed.ncbi.nlm.nih.gov/16525181/
- White HH. Brain stem tumors occurring in adults. Neurology. 1963; 13: 292–300. https://pubmed.ncbi.nlm.nih.gov/14000317/
- Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001; 124: 2528–2539. https://pubmed.ncbi.nlm.nih.gov/11701605/
- Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998; 40: 265–271. https://pubmed.ncbi.nlm.nih.gov/9457808/
- Fischbein NJ, Prados MD, Wara W, Russo C, Edwards MS, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996; 24: 9–23. https://pubmed.ncbi.nlm.nih.gov/8817611/
- Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 2006; 7: 241–248. https://pubmed.ncbi.nlm.nih.gov/16510333/
- Wagner S, Warmuth-Metz M, Emser A, Gnekow AK, Strater R, et al. Treatment options in childhood pontine gliomas. J Neuro-oncol. 2006; 79: 281–287. https://pubmed.ncbi.nlm.nih.gov/16598416/
- Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol. 2014; 16: iv1–63. https://pubmed.ncbi.nlm.nih.gov/25304271/
- Registre des Cancers de la Région du Grand Casablanca 2008- 2012 n.d. Cancer Incidence and Survival Among Children and Adolescents - Pediatric Monograph – SEER Publications. 1975- 1995.
- Truffaux N. Nouvelles cibles thérapeutiques dans les gliomes infiltrants du tronc cérébral de l’enfant. Médecine humaine et pathologie Université Paris Sud - Paris XI, 2014.
- González OE, Casas C, Bermú dez YM. State of the art: pediatric brain stem gliomas. Revista Colombiana de Cancerología 2017; 21: 202–211.
- Epstein F, Constantini S. Practical decisions in the treatment of pediatric brain stem tumors. Pediatr Neurosurg 1996; 24: 24–34. https://pubmed.ncbi.nlm.nih.gov/8817612/
- Sun B, Wang CC, Wang J. MRI characteristics of midbrain tumours. Neuroradiology. 1999; 41: 158–162. https://pubmed.ncbi.nlm.nih.gov/10206156/
- Barkovich AJ, Krischer J, Kun LE, Packer R, Zimmerman RA, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990; 16: 73–83. https://pubmed.ncbi.nlm.nih.gov/2132928/
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica. 2016; 131: 803–820. https://pubmed.ncbi.nlm.nih.gov/27157931/
- Garibotto F, Madia F, Milanaccio C, Verrico A, Piccardo A, et al. Pediatric Diffuse Midline Gliomas H3 K27M- Mutant and Non-Histone Mutant Midline High-Grade Gliomas in Neurofibromatosis Type 1 in Comparison With Non-Syndromic Children: A Single-Center Pilot Study. Front Oncol. 2020; 10: 795. https://pubmed.ncbi.nlm.nih.gov/32582540/
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary Neuro-Oncology. 2021; 23: 1231–1251. https://pubmed.ncbi.nlm.nih.gov/34185076/
- Schild SE, Stafford SL, Brown PD, Wood CP, Scheithauer BW, et al. The results of radiotherapy for brainstem tumors. J Neurooncol. 1998; 40: 171–177. https://pubmed.ncbi.nlm.nih.gov/9892099/
- Albright AL, Guthkelch AN, Packer RJ, Price RA, Rourke LB. Prognostic factors in pediatric brain-stem gliomas. J Neurosurg. 1986; 65: 751–755. https://pubmed.ncbi.nlm.nih.gov/3772472/
- Schulz-Ertner D, Debus J, Lohr F, Frank C, Höss A, et al. Fractionated stereotactic conformal radiation therapy of brain stem gliomas: outcome and prognostic factors. Radiother Oncol. 2000; 57: 215–223. https://pubmed.ncbi.nlm.nih.gov/11054526/
- Khan L, Soliman H, Sahgal A, Perry J, Xu W, et al. External beam radiation dose escalation for high grade glioma. Cochrane Database of Syst Rev. 2020; 5: CD011475. https://pubmed.ncbi.nlm.nih.gov/32437039/
- Hu X, Fang Y, Hui X, Jv Y, You C. Radiotherapy for diffuse brainstem glioma in children and young adults. Cochrane Database Syst Rev. 2016: CD010439. https://pubmed.ncbi.nlm.nih.gov/27378212/
- Wang S, Zheng D, Zhang C, Ma R, Bennion NR, et al. Automatic planning on hippocampal avoidance whole-brain radiotherapy. Med Dosim. 2017; 42: 63–68. https://pubmed.ncbi.nlm.nih.gov/28237294/
- Laprie A, Hu Y, Alapetite C, Carrie C, Habrand JL, et al. Radiotherapycommittee of SFCE and France Hadron Paediatric brain tumours: A review of radiotherapy, state of the art and challenges for the future regarding protontherapy and carbontherapy. Cancer Radiother. 2015; 19: 775-789. https://pubmed.ncbi.nlm.nih.gov/26548600/
- Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low- grade gliomas. Int J Radiat Oncol Biol Phys. 2014; 89: 1060–1068. https://pubmed.ncbi.nlm.nih.gov/25035209/
- Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012; 30: 2641–2647. https://pubmed.ncbi.nlm.nih.gov/22665535/
- Gururangan S, Fangusaro J, Poussaint TY, McLendon RE, Onar-Thomas A, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014; 16: 310–317. https://pubmed.ncbi.nlm.nih.gov/24311632/
- Nicholson HS, Kretschmar CS, Krailo M, Bernstein M, Kadota R, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer 2007; 110: 1542–1550. https://pubmed.ncbi.nlm.nih.gov/17705175/