More Information
Submitted: December 22, 2021 | Approved: January 17, 2022 | Published: January 18, 2022
How to cite this article: Riccioni L, Balestrieri A, Dalila F, Nasi MT, Tosatto L. Intrasellar psammomatous meningioma: a case report and review of the literature. J Neurosci Neurol Disord. 2022; 6: 011-015.
DOI: 10.29328/journal.jnnd.1001061
Copyright License: © 2022 Riccioni L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Meningioma; Intrasellar; Psammomatous; Pituitary adenoma; Transsphenoidal surgery
Intrasellar psammomatous meningioma: a case report and review of the literature
Luca Riccioni1* , Antonio Balestrieri2, Fuschillo Dalila3,Maria Teresa Nasi3 and Luigino Tosatto3
, Antonio Balestrieri2, Fuschillo Dalila3,Maria Teresa Nasi3 and Luigino Tosatto3
1OU of Anatomic Pathology, Maurizio Bufalini Hospital, Cesena (FC), Italy
2Endocrinology and Diabetology Unit, Maurizio Bufalini Hospital, Cesena (FC), Italy
3OU of Neurosurgery, Maurizio Bufalini Hospital, Cesena (FC), Italy
*Address for Correspondence: Riccioni Luca, OU of Anatomic Pathology, Maurizio Bufalini Hospital, Viale Ghirotti 286, 47521 Cesena (FC), Italy, Email: [email protected]
Intrasellar meningioma (IM) is a rare occurrence that is difficult to distinguish preoperatively from the most common non-functioning pituitary adenoma. Here we describe a case of psammomatous IM occurring in a 68-year-old woman, presented with visual defects. On magnetic resonance imaging (MRI) she was found to have an intrasellar mass with suprasellar extension that was approached with transsphenoidal surgery. Subtle radiological hints, namely dural tail sign, intralesional calcifications and a marked and homogenous early enhancement of IM on MRI after gadolinium administration, may aid clinicians in achieving an accurate pre-operative diagnosis and choosing the proper surgical approach. The clinical and neuroradiological features of IM described in the literature has been reviewed.
Meningiomas are the most common intracranial tumors, comprising approximately 32% of all central nervous system (CNS) related neoplasms [1]. They arise from the arachnoid cap cells and may occur anyway in the neuraxis, although they show a predilection for the cerebral convexities, parasagittal areas and the sphenoid ridge. Meningiomas of suprasellar and parasellar region account for 5% - 10% of all intracranial meningiomas [2-4], whilst those with a pure intrasellar locations represent only 1% of sellar masses [5,6]. They mimic clinically and radiologically non-functioning pituitary macroadenomas. So a correct preoperative diagnosis is of great importance because it allows a safe surgical approach and a complete tumour resection. Here we present an additional case of intrasellar meningioma (IM) and a review of the pertinent literature is provided.
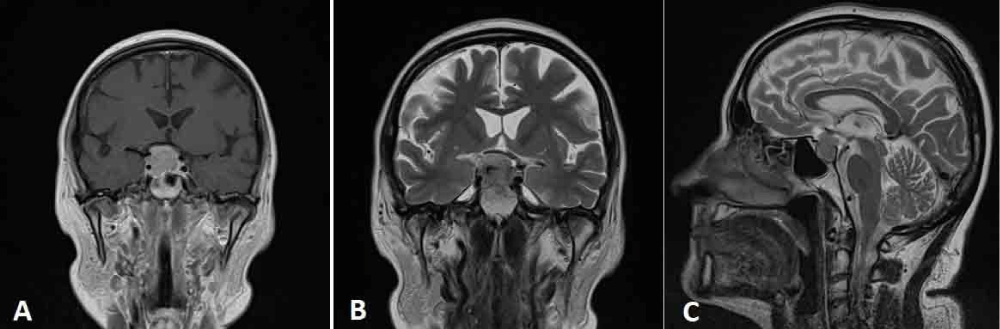
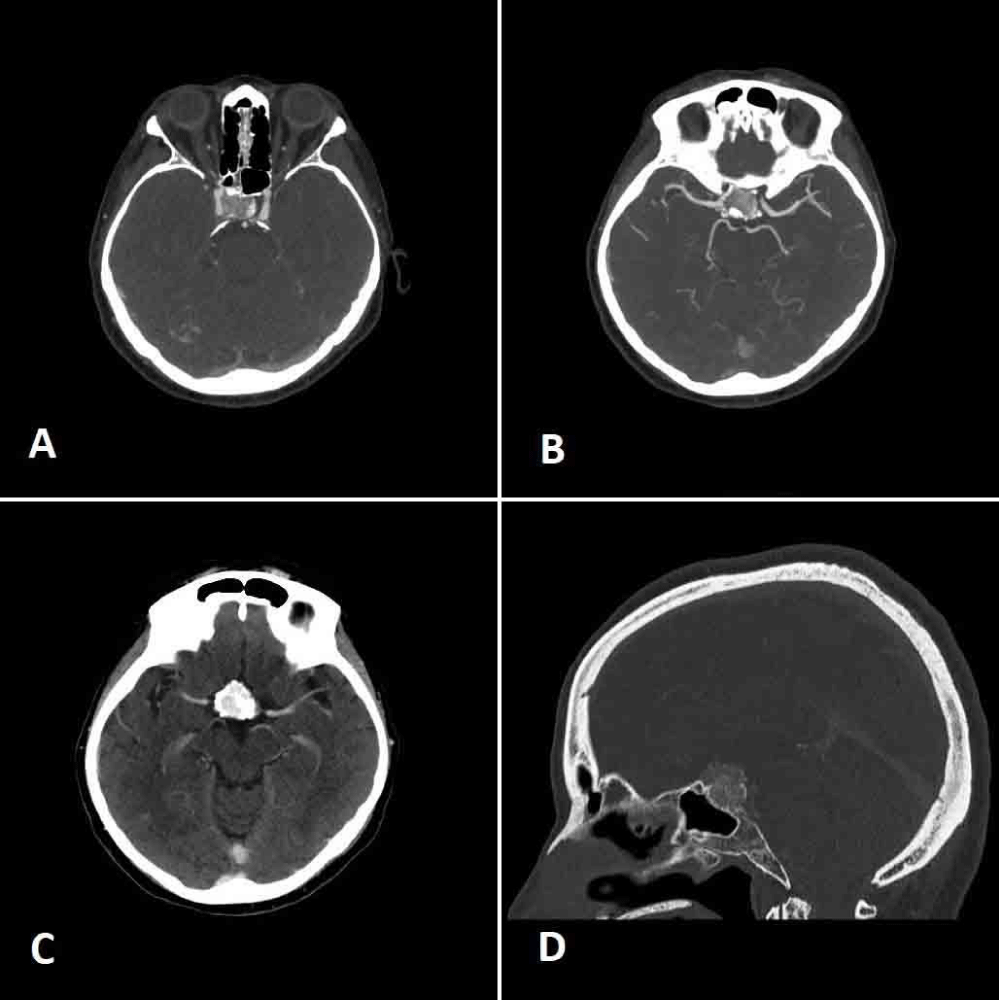
A 68-year-old woman was admitted to the Neurosurgery Department of the “M. Bufalini” Hospital, because of a visual field defect of the upper outer quadrant of the right eye. An enhanced magnetic resonance imaging (MRI) revealed the presence of an expansive intrasellar mass measuring 20 x 24.8 mm with suprasellar extension and calcifications on its left side. It caused the compression of the optic chiasm, mainly on the right side and the depression of the right sellar floor and showed the invasion of the right cavernous sinus and (Figure 1). Preoperative angio-computed tomography (CT) (Figure 2) showed an early and intense contrast enhancement and confirmed the right carotid artery encasement. Anyway, it was patent with a narrow diameter. The pituitary gland was not clearly identified. Preoperative hormonal tests revealed a state of hypopituitarism characterized by low level of plasma cortisol (45 mcg/l, normal value - n.v. 62–180) with an inappropriate normal level of ACTH (20 ng/l n.v. : 7,2 – 63 ng/l); low level of Ft4 (7 ng/ml ; n.v. : 8- 16 ng/ml) with an inappropriate normal level of TSH (1,1 mui/l); undetectable levels of estradiol (< 18 pmol/l) and of LH (< 0.3 UI/L) associated with low level of FSH (1,1 UI /l) that documented an inappropriate gonadal setting for the age of the patient. A modestly high level of prolactin was also documented (68,7; v.n. < 24 mcg/l). The patient was treated with hydrocortisone acetate 25 mg/day and L-thyroxine 50 mcg/day.
Figure 1: A: coronal preoperative post contrast MRI; B and C: coronal and sagittal T2 weighted images demonstrating the sellar lesion with suprasellar extension and invasion of the right cavernous sinus.
Figure 2: A. and B. Axial angio-CT scan demonstrating the encasement of the right carotid artery, the presence of calcifications and the moderate sellar enlargement; C. axial CT scan showing the early and marked contrast enhancement of the mass; D. sagittal CT scan showing the thinning of the sellar floor due to the expansive mass.
The clinical-radiological data suggested firstly a non-secreting pituitary macroadenoma, but a meningioma or a craniopharyngioma was considered as well. Surgery was performed with a transsphenoidal approach and the tumour was gross totally removed and tissue was sent for intraoperative frozen section and for routine histological examination.
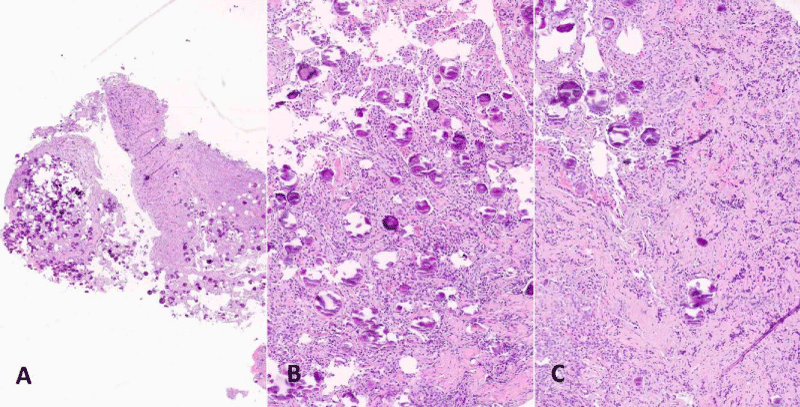
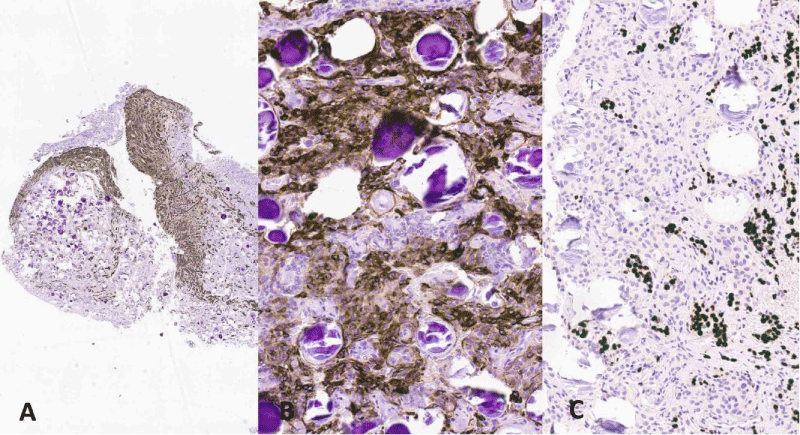
Tissue was fixed in 10% buffered formalin, embedded in paraffin blocks and sections were routinely stained with Haematoxylin and Eosin (H&E). Histologically the tumour was composed of polygonal meningothelial cells arranged in vorticoid nests. Nuclei were monomorphic, frequently with pseudoinclusions. There were numerous psammomatous calcifications, in some areas preponderant (Figure 3). Neither mitoses nor necrosis were present. Fragments of slightly fibrotic adenohypophysis were presents admixed with the neoplastic tissue. On immunohistochemistry (Figure 4) tumour cells were positive with EMA (Epithelial Membrane Antigen) and Somatostatin receptor and negative for Chromogranin, GATA 3, PIT1 (pituitary transcription factor 1) and TPIT (T-box transcription factor). The latter four antibodies as well as antibodies against pituitary hormones were consistently expressed in the adenohypophyseal tissue. The ki67 proliferation index was 2%.
Figure 3: (H&E): Section at low power magnification shows the tumour rich in psammomatous bodies attached to residual adenohypophyseal tissue (A, original magnification x200). Tumour is composed of polygonal meningothelial cells admixed with psammomatous calcifications (B, x200), infiltrating remnants of the pituitary gland on the left side (C, original magnification x200).
Figure 4: (ICH): the residual adenohypophyseal tissue is highlighted by Chromogranin antibody (A, original magnification x200). Tumour cells are positive for Epithelial membrane antigen (EMA) (B, x200). Nests of pituitary gland positive with PIT1 (nuclear immunostain) are mingled with the tumour cells (C, x200).
The morphological and immunohistochemical data were consistent with a Psammomatous Meningioma, grade I according to WHO classification [1].
A transient phase of diabetes insipidus (polyuria and polydipsia) was documented for a few days after surgery and corrected with a small dosage of sublingual desmopressin (60 mcg/day). Hypopituitarism did not recover and the woman was discharged with hydrocortisone and L- thyroxine.
The most frequent causes of incidentally detected sellar region masses are pituitary adenomas, followed by craniopharyngiomas, cystic non-neoplastic lesions, infla-mmatory lesions, meningiomas and metastases. Skull base meningiomas occur more frequently in the sphenoid ridge and tuberculum sellae and rarely present with a pure intrasellar location, with only 19 single cases reported to date [2-4,7-16]. They occur most commonly in middle-aged and elderly patients and more frequently in females, with a reported F:M ratio of 6:1 [5,6]. Symptoms of intrasellar meningiomas generally develop gradually [2], mainly due to compression of the optic nerve, or pituitary gland. They include more frequently visual disturbances and headaches, whilst very few patients initially manifest clinical, or endocrine hormonal abnormalities. The latter include endocrine manifestation of hypopituitarism and hyperprolactinemia due to stalk compression [5,6]. Rarely sudden onset of symptoms has been reported. They may intervene with intratumor haemorrhage, mimicking pituitary apoplexy [7,11].
Here we describe a rare case of IM that presented as an expansive mass with suprasellar extension. The lesion was preoperatively considered more in keeping with a non-functional pituitary macroadenoma and a transsphenoidal approach was preferred. Anyway, the presence of an early, marked and homogeneous contrast enhancement on angio-TC, as well as the presence of calcifications, raised doubts about the preoperative diagnosis. An intraoperative frozen section revealed that the tumour was a psammomatous meningioma. The difficult preoperative diagnosis can be explained by histology, which showed the neoplasm was tightly attached to the pituitary gland, which appeared compressed and fibrotic and not discernible on radiological exams, as well as intraoperatively. So the complete removal of the tumour led to consequent hypopituitarism.
Meningiomas develop more frequently in the parasellar or suprasellar location. According to Kinjo, et al. [17] tuber-culum sellae and diaphragmatic meningiomas are separate entities. The latter have been further distinguished by Kinjo, et al. in three distinct subtypes, according to their site of origin from diaphragm: type A, supradiaphragmatic-prepituitary; type B, supradiaphragmatic-retropituitary; type C, subdiaphragmatic. This subtype originates from the bottom surface of the diaphragm sellae and frequently shows clinical and radiological features similar to macroadenomas. Intrasellar meningioma can arise also from the dura covering the wall and the floor of the sella turcica. These sub-glandular meningioma are extremely rare with only 5 cases reported in the literature [2,7,8,15].
A different radiologic classification approach considering the supradiaphragmatic (suprasellar) and infradiaphragmatic (sellar) heights has been more recently proposed by Ajlan, et al. [18]. These authors identified on T1-weighted MRI imaging three osseous points (P - posterior planum point; T – tuberculum; C -clival-sellar floor point), that allowed the measurement of the planum-tuberculum interval (PTI), the tuberculum-sellar floor interval (TSFI) and the total sellar height (TH: PTI+TSFI). They recognized three different patterns of tumour growth. Type A: true tuberculum sellae meningiomas, associated with elongation of the PTI. They present without anterior intercavernous sinus (AIS) involvement and are prone to complete surgical resection, through a purely supradiaphragmatic endoscopic approach. Type B: TS-DS meningiomas, associated with elongation (not statistically significant) of either PTI and TSFI. They present frequently involvement of the diaphragm sellae and AIS and require a supra-and infra-diaphragmatic access for a complete resection of the tumour. Type C: true diaphragma sellae meningiomas.
A correct preoperative diagnosis of sellar meningioma is important because it allows better surgical planning. In fact, suprasellar meningioma are preferentially approached via craniotomy. Diagnostic clues that facilitate the differential diagnosis between sellar meningioma and pituitary adenoma include the presence of dural tail sign, calcifications and homogeneous and marked rapid contrast enhancement of the mass [2]. The latter feature seems to be the best guide for the differential diagnosis, as “dural tail” is not typical of intrasellar meningioma, being preferentially described in the suprasellar tumours. Calcifications are not typical of pituitary adenomas. Even if their presence does not exclude an adenoma, they are more often present in intrasellar meningiomas, aneurysms, and craniopharyngiomas. Bright homogeneous and rapid enhancement with gadolinium on MRI is a characteristic finding in more than 90% of sellar meningioma, while pituitary adenoma generally shows a heterogeneous and less intense enhancement. Anyway, this is not the rule because the presence of a prevalent stromal component as in fibrous meningioma, may favour a slow increment without a peak of contrast enhancement. Cappabianca, et al. [19] in their series, for differential diagnosis pointed to the prevalent suprasellar growth and moderate sellar enlargement of diaphragma sellae meningioma. At different non-functional pituitary macroadenoma are associated with marked sellar enlargement.
The majority of sellar meningiomas described in literature are grade I of the meningothelial, transitional and fibrous type, with only three cases of the psammomatous variant reported to date [6,9]. According to the WHO classification [1] this subtype is characterized by the presence of numerous psammomatous calcifications, that sometimes overwhelm the meningothelial cellular component. Less frequently grade II atypical mengiomas, showing the defining features of the Who classification, or pertaining to the clear cell variant have been reported [16]. Even a unique case of a grade III malignant diaphragm meningioma has been described [4]. Grade I meningiomas can be easily differentiated from pituitary adenoma, when a syncytial growth, whorls and intervening fibroblastic elements are present. Psammoma bodies are typical of transitional and psammomatous meningiomas, but not exclusive being present also in some prolactin and thyrotropin-producing adenomas. Anyway when a meningothelial, or an atypical subtype is suspected the recourse to immunohistochemistry is advisable. At difference with pituitary neuroendocrine tumours, meningiomas stain with EMA and Progesterone receptor but do not express neuroendocrine markers as Chromogranin or Synaptophysin. Somatostatin receptor is a robust immunohistochemical
marker for meningiomas, but it is not very useful in such cases, being intensely positive also in normal adenohypophyseal tissue as well as in pituitary adenoma.
The correct surgical approach is critical for the management of sellar meningiomas in order to resect as much tumour as possible, to prevent intraoperative bleeding and preserve the surrounding critical neurovascular structures. Transcranial approach has been traditionally reserved to tuberculum sellae and suprasellar (Kinjo’s type A and type B) meningiomas, while the transsphenoidal approach has been preferred as first choice of approach for Kinjo’s type C/intrasellar meningioma, also with suprasellar extension [15,17,20,21]. It appears as a relatively safe route for intrasellar masses regardless of their clinic-pathological diagnosis. This approach is well tolerated by patients and allows either pituitary decompression and confirmation of the diagnosis, or a radical removal of IM [12]. Contraindications for the transsphenoidal approach include large tumor size, encasement of the internal carotid artery and difficulty in removing the fibrous tumor with poor control of the surrounding neurovascular structures. A two-staged, or a combined transsphenoidal and transcranial approach has been respectively adopted by Kinjo, et al. [17] and Nozaki, et al. for large intrasellar and suprasellar meningiomas [8,22,23]. According to the large series of purely or largely intrasellar meningiomas reported by Sayhananthan et al. (6), the residual tumour was documented in 6 out 17 patients (35%) and only three of them experienced a progression of the residual meningioma. Additional two cases recurred respectively at 6 and 13 years from the first excision underwent surgery again with frontotemporal and bifrontal craniotomy. Adjunctive external beam irradiation (total dose of 5.400 cGy in 30 fractions in all cases) was administered for residual disease in five of their patients. Postoperative radiation has been rarely adopted, targeting the residual or recurrent tumour and in the rare cases of grade II (atypical) and grade III (anaplastic/malignant) meningiomas. The malignant meningioma described by Zhou, et al. [4] required for treatment also three cycles of combined chemotherapy with cyclophosphamide and nimustine, with no evidence of tumour regrowth at 3-year follow-up.
In conclusion, intrasellar meningioma is a rare cause of intrasellar mass that may be difficult to differentiate from a non-functioning pituitary adenoma. The correct pre-operative diagnosis needs to take account of clinical presentation, hormonal and radiological data, as a rapid rise and gradual decline of the enhancement curve on MRI. Anyway, the differential diagnosis remains still difficult and the transsphenoidal approach appears as the most feasible and preferable route for surgical treatment of doubtful cases of intrasellar mass.
Standard of reporting: CARE guidelines and methodo-logies have been followed.
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval and Informed Consent: Not applicable.
Human and animal rights: All human procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
Standard of reporting: CARE guidelines and methodologies have been followed.
All the authors equally contributed to the evaluation of the case reports. Luca Riccioni made histological diagnosis at time of surgery and wrote the paper. Antonio Balestrieri and Dalila Fuschillo were involved in the clinical and surgical management and partially wrote the paper. Maria Teresa Nasi and Luigino Tosatto performed the transsphenoidal surgery. All the Authors made critical revision and gave final approval of the manuscript.
Trial registration
Not applicable, because this article does not contain any clinical trials.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, et al. WHO classification of tumour of the central nervous system 4th ed, 2016.
- Wang CJ, Howng SL, Huang JS. Intrasellar meningioma. Kaohsiung J Med Sci. 1996; 12: 486-490.
- Bang M, Suh JH, Park JB, Weon YC. Pure intrasellar meningioma mimicking pituitary macroadenoma: magnetic resonance imaging and review of the literature. World Neurosurg. 2016; 675: E1-4. PubMed: https://pubmed.ncbi.nlm.nih.gov/27126911/
- Zhou P, Yin S, Jiang S, Cai B. Malignant intrasellar meningioma presenting as an invasive pituitary macroadenoma: a rare case report and literature review. Oncol Lett. 2016; 11: 1073-1076. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4733968/
- Kwancharoen R, Blitz Ari M, Tavare SF, Caturegli P, Gallia Gary L, et al. Clinical features of sellar and suprasellar meningiomas. Pituitary. 2014; 17: 342-348. PubMed: https://pubmed.ncbi.nlm.nih.gov/23975080/
- Sathananthan M, Sathananthan A, Scheithauer BW, Giannini C, Meyer FB, et al. Sellar meningiomas: an endocrinologic perspective. Pituitary. 2013; 16: 182-188. PubMed: https://pubmed.ncbi.nlm.nih.gov/22644157/
- Kudo H, Takaishi Y, Minami H, Takamoto T, Kitazawa S, et al. Intrasellar meningiomas mimicking pituitary apoplexy. Surg Neurol.1997; 48: 374-381. PubMed: https://pubmed.ncbi.nlm.nih.gov/9315136/
- Nozaki K, Nagata I, Yoshida K, Kikuchi H. Intrasellar meningioma: case report and review of the literature. Surg Neurol. 1997; 47: 447-452. PubMed: https://pubmed.ncbi.nlm.nih.gov/9131027/
- Yoneoka Y, Tanaka R, Minakawa T, Tamura T. Psammomatous meningioma arising from the diaphragma sellae. Acta Neurochi (Wien). 1998; 140: 291-292. PubMed: https://pubmed.ncbi.nlm.nih.gov/9638269/
- Matsumoto S, Hayase M, Inamura H, Oda Y, Kikuchi H, et al. A case of intrasellar meningioma mimicking pituitary adenoma. No Shinkei Geka. 2001; 29: 551-557. PubMed: https://pubmed.ncbi.nlm.nih.gov/11452502/
- Orakdogeny M, Karadereler S, Berkman Z, Ersahin M, Ozdogan C, et al. Intra-suprasellar meningioma mimicking pituitary apoplexy. Acta neurochir (Wien). 2004; 146: 511-515. PubMed: https://pubmed.ncbi.nlm.nih.gov/15118889/
- Pinzer TH, Krishnan KG, Schackert G. The diaphragm sellae meningioma – a rare differential diagnosis of Non-Functioning Pituitary adenoma. Zentralbl Neurochir. 2004; 65: 195-197. PubMed: https://pubmed.ncbi.nlm.nih.gov/15551185/
- Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningioma of atypical (WHO grade II) histology. J Neurooncol. 2010; 99: 393-405. PubMed: https://pubmed.ncbi.nlm.nih.gov/20740303/
- Barrera JR, Carpio EJT, Pena CMS. Giant sellar meningioma mimicking pituitary macroadenoma. BMJ Case Reports. 2012; 2012: bcr2012006703. PubMed: https://pubmed.ncbi.nlm.nih.gov/22949000/
- Cha SW, Park DW, Park CK, Lee YJ. Pure intrasellar meningioma located under the pituitary gland: case report. Korean J Radiol 2013; 14: 321-323. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3590347/
- Yin S, Zhou P, LI Q, Jiang S. Intrasellar clear cell meningioma mimicking invasive pituitary adenoma: a case report and review of the literature. Turk Neurosurg. 2015; 25: 976-979. PubMed: https://pubmed.ncbi.nlm.nih.gov/26617154/
- Kinjo T, Al-Mefty O, Ciric I. Diaphragma sellae meningiomas. Neurosurgery. 1995; 36: 1082-1092. PubMed: https://pubmed.ncbi.nlm.nih.gov/7643985/
- Ajlan AM, Choudhri O, Hwang P, Harsh G. Meningioma of the tuberculum and diaphragm sellae. J neurol Surg B. 2015; 76: 74-79. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318732/
- Cappabianca P, Cirillo S, Alfieri A, D’Amico A, Maiuri F, et al. Pituitary macroadenoma and diaphragma sellae meningioma: differential diagnosis on MRI. Neuroradiology. 1999; 41: 22-26. PubMed: https://pubmed.ncbi.nlm.nih.gov/9987763/
- Honnegger J, Fahlbusch R, Buchfelder M, Huk WJ, Thierauf P. The role of transsphenoidal microsurgery in the management of sellar and parasellar meningioma. Surg Neurol. 1993; 39: 18-24. PubMed: https://pubmed.ncbi.nlm.nih.gov/8451714/
- Civit T, Marchal JC, Pinelli C, Auque J, Hepner H. [Meningiomas of the sellar diaphragm. Apropos of 4 cases*. Neurochirurgie. 1997; 43: 21-27. PubMed: https://pubmed.ncbi.nlm.nih.gov/9205623/
- Bulsara KR, Essayed WI, Aboud E, Al-Mefty O. Diaphragm sellae meningioma: distinct clinical, anatomic, and surgical considerations: 2-dimensional operative video. Operative Neurosurg. 2021; 21: E336-E337. PubMed: https://pubmed.ncbi.nlm.nih.gov/34171924/
- Belouaer A, Starnoni D, Daniel RT. Surgery for diaphragm sellae meningioma: how I do it. Acta Neurochirurgica. 2021; 163: 97-100. PubMed: https://pubmed.ncbi.nlm.nih.gov/32945959/