More Information
Submitted: February 07, 2022 | Approved: May 09, 2022 | Published: May 10, 2022
How to cite this article: Erdoğan HA, Yayla V, Sözer N, Aydın FY, Acır I, et al. Idiopathic parkinson’s disease and fatigue. J Neurosci Neurol Disord. 2022; 6: 016-019.
DOI: 10.29328/journal.jnnd.1001062
Copyright License: © 2022 Erdoğan HA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Idiopathic parkinson’s disease; Fatigue
Idiopathic parkinson’s disease and fatigue
Hacı Ali Erdoğan1, Vildan Yayla1, Nejla Sözer1, Filiz Yıldız Aydın2, Ibrahim Acır1* and Meltem Vural2
and Meltem Vural2
1Neurology Department, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey
2Physical Medicine and Rehabilitation Department, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey
*Address for Correspondence: Ibrahim Acır, Neurology Department, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey, Email: [email protected]
Introduction: Fatigue is a common non-motor symptom of Idiopathic Parkinson’s Disease (IPD). The aim is to research the relationship between fatigue of IPD patients and the clinical findings, of mood disorders.
Material and methods: A total of 39 patients with IPD were included in the study. The relationship between fatigue severity and demographic characteristics and the treatment was evaluated in IPD. The severity of fatigue was evaluated by Fatigue Severity Scale (FSS). Motor impairment was scored by the modified Hoehn and Yahr scale. The patients were assessed for the presence of depression and anxiety with the Hospital Anxiety and Depression Scale (HADS).
Results: The mean age of the patients was 70.62 ± 8.35 years. 23 were men and 16 were women. The mean disease duration was 6.18 ± 3.35 years. The patients were assigned into two groups according to the presence of fatigue measured by FSS with less than 5 (Group I) and 5 or more (Group II). There were no statistically significant differences between the two groups with respect to mean age, mean age of onset, and mean disease duration of the patients (p > 0.05). There were no significant differences between the two groups for HADS depression, anxiety values, and terms of antiparkinsonian therapies (p > 0.05). The severity of fatigue was correlated with the HADS anxiety levels (p < 0.05).
Discussion: Fatigue is an important non-motor symptom that is underestimated in clinical follow-up. We didn’t find any correlation between fatigue and age, duration of disease onset, or drug use. There was no significant correlation between the fatigue score and depression, and pain. However, the fatigue scores were higher in patients with high anxiety scores and females.
Idiopathic Parkinson’s Disease (IPD) is the second most common neurodegenerative disorder causing disability [1]. The main motor clinical manifestations of IPD are resting tremor, bradykinesia, rigidity, and postural instability. Mostly underestimated non-motor symptoms may cause severe disability [2.3]. Fatigue which is defined as a lack of energy and exhaustion is the most common non-motor manifestation of IPD.
Many different scales are used for objective measurement and evaluation of fatigue. The relationship between fatigue and demographic data, clinical characteristics, and the treatment is important for individual rehabilitation strategies to be applied to patients and also can be a guide for new rehabilitation strategies. The aim of our study is to determine the relationship between fatigue and demographic characteristics, disease severity, depression, anxiety, pain, and drug therapy.
A total of 39 patients with Parkinson’s Disease who were followed up at our Movement Disorders Outpatient Clinics were included in the study. The severity of fatigue was evaluated by the fatigue severity scale (FSS). The Hoehn-Yahr scale was used to evaluate the severity of the disease and the Hospital Anxiety and Depression Scale (HADS) for the presence of anxiety or depression. Fatigue was evaluated according to the FSS. If FSS>5, that means ‘fatigue positive’. If FSS<5, then it means ‘fatigue negative’. Anxiety and depression were classified as mild (0-7), moderate (8-10), and severe (11-21) according to the HADS. Neuropathic pains of the patients were evaluated with Douleur Neuropathique en 4 Questions (DN4). Patients with severe hearing problems, moderate to severe cognitive impairment, and systemic diseases which can prevent cooperation were excluded.
Statistical analysis
For the statistical analysis, NCSS (Number Cruncher Statistical System) 2007 (Kaysville, Utah, USA) program was used. In the evaluation of the data, descriptive statistical methods (Mean, Standard Deviation, Minimum, Maximum) as well as the independent sample T-Test for parametric samples and Mann Whitney U test for nonparametric samples. In the comparison of qualitative data, Fisher’s exact test and Yates’ Continuity Correction test were used. Spearman’s Correlation Analysis was used to evaluate the relationships between the samples. Significance was evaluated at p < 0.05 levels.
A total of 39 patients (23 male and 16 female) aged between 50-82 years (mean: 70.62 ± 8.35 years) were included in the study, 59% (23) of the cases were male and 41% (16) were female.
There were no statistically significant differences between the mean age of the patients with fatigue, the age of onset of the disease, and the duration of the disease with fatigue.
Fatigue in female patients was significantly higher than that of males (p = 0.007; p < 0.01).
No statistically significant differences were found between the use of anti-parkinsonian drugs (MAO-B inhibitor, Dopa agonist, L-Dopa), antidepressant, and antipsychotic medication with the presence of fatigue (p > 0.05) (Table 1).
| Table 1: Evaluation of descriptive properties according to fatigue presence. | ||||
| Fatigue(-) (n = 21) | Fatigue(+) (n = 18) | p | ||
| Mean ± SD | Mean ± SD | |||
| Age (years) | 70.57 ± 7.02 | 70.67 ± 9.89 | a0.972 | |
| Age at onset of disease (years) | 64.38 ± 7.59 | 64.50 ± 9.70 | a0.966 | |
| Disease duration (years); (Median) | 6.19 ± 3.68 (5) | 6.17 ± 3.03 (6) | b0.744 | |
| n (%) | n (%) | |||
| Gender | Female | 4 (19.0) | 12 (66.7) | c0.007** |
| Male | 17 (81.0) | 6 (33.3) | ||
| MAO-B inhibitor | None | 8 (38.1) | 2 (11.1) | d0.074 |
| Yes | 13 (61.9) | 16 (88.9) | ||
| Dopa agonist | None | 5 (23.8) | 5 (27.8) | d1.000 |
| Yes | 16 (76.2) | 13 (72.2) | ||
| L-DOPA | None | 5 (23.8) | 2 (11.1) | d0.418 |
| Yes | 16 (76.2) | 16 (88.9) | ||
| Antidepressant | None | 20 (95.2) | 14 (77.8) | d0.162 |
| Yes | 1 (4.8) | 4 (22.2) | ||
| Antipsychotic | None | 20 (95.2) | 15 (83.3) | d0.318 |
| Yes | 1 (4.8) | 3 (16.7) | ||
| aStudent t Test, bMann Whitney U Test. cYates’s Continuity Correction Test, dFisher’s Exact Test, **p < 0.01. | ||||
There was no statistically significant correlation between fatigue and Hoehn-Yahr scale, neuropathic pain, and depression (Table 2).
| Table 2: Relation between fatigue score and neuropathic pain, Hoehn-Yahr, anxiety and depression scores. | ||
| Fatigue score | ||
| r | p | |
| Hoehn-Yahr | 0.112 | 0.497 |
| Neuropathic pain | 0.245 | 0.132 |
| Anxiety | 0.377 | 0.018* |
| Depression | 0.134 | 0.417 |
| r: Spearman’s Correlation Coefficient *p < 0.05 | ||
There was no significant correlation between fatigue and age, duration of disease onset, drug use, depression, and pain. As a positive finding, the fatigue scores were higher in patients with high anxiety scores and females.
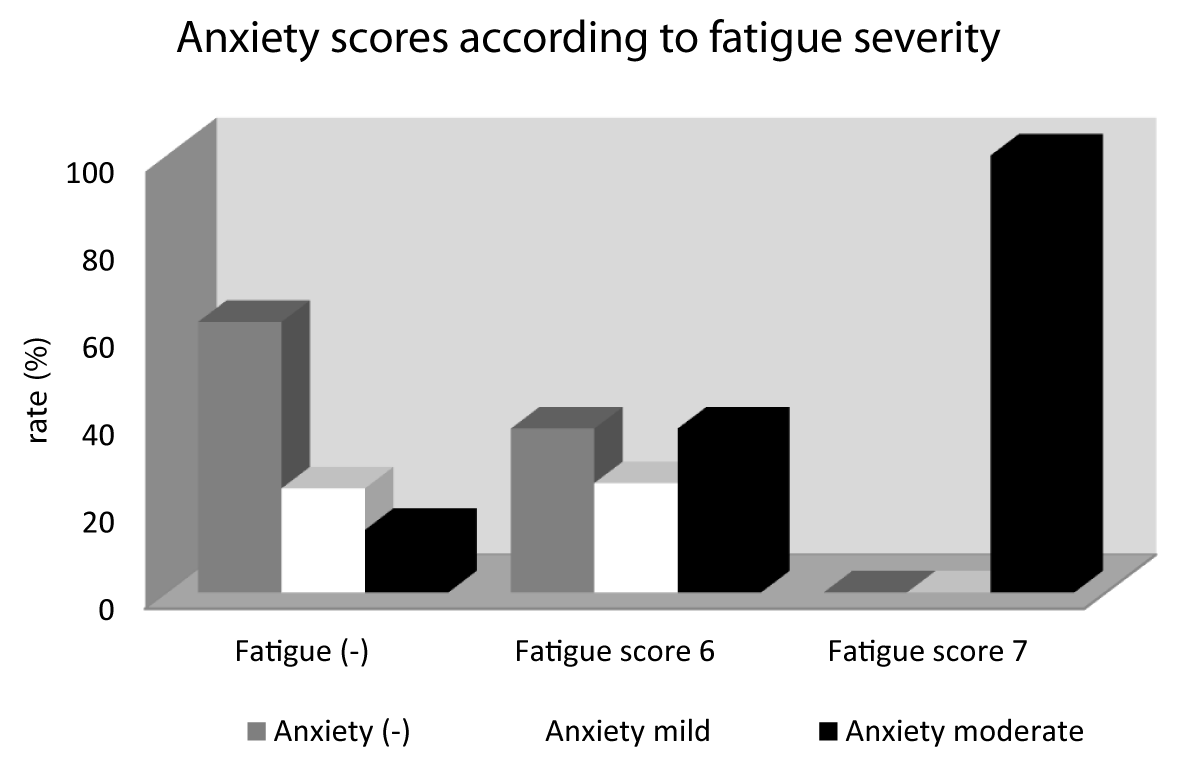
There was a statistically significant correlation between anxiety scores and fatigue scores (r: 0.377; p: 0.018) (Figure 1).
Figure 1: A: coronal preoperative post contrast MRI; B and C: coronal and sagittal T2 weighted images demonstrating the sellar lesion with suprasellar extension and invasion of the right cavernous sinus.
IPD is a progressive neurodegenerative disorder characterized by prominent motor and non-motor symptoms. Non-motor symptoms such as autonomic symptoms, sleep, neuropsychiatric disorders, and fatigue are as important as motor symptoms [4]. In the last two decades, non-motor findings have gained more importance in IPD. These non-motor findings, which are mostly overlooked in routine patient controls, play important role in disability [5]. Fatigue rates were reported at 33% - 58% in different IPD patient populations and it was accepted as the most important non-motor symptom [6,7)] In a study by Friedman et al. one-third of IPD patients reported fatigue as the most important cause of disability, with more than half showing fatigue among the three leading causes of disability [8]. In our study, fatigue was found in 18 (46%) of 39 patients who underwent FSS.
Fatigue complaints were generally not associated with the severity of motor symptoms [9]. Therefore, it does not respond to dopaminergic and surgical treatment [10-12]. Many studies reported that fatigue severity increases in patients with advanced Hoehn-Yahr scores [13,14]. As in the study of Abe et al, we found no significant relationship between the Hoehn-Yahr score and fatigue [15]. In many studies, there was no association between demographic characteristics and fatigue, whereas in some studies fatigue was more common in female patients. In our patients, there was a statistically significant correlation between the female gender and the presence of fatigue. As in the literature, we didn’t find any correlation between age, duration of disease onset, drug use, and the presence of fatigue [16-20]. The relationship between neuropsychiatric symptoms and fatigue has not been clarified. In some studies, a positive correlation was found between depression and fatigue while in other studies weren’t found any correlation [8,15].
In our study, we found, that there was no significant correlation between the fatigue score and depression and pain. However, the fatigue scores were higher in patients with high anxiety scores.
The small number of patients (n: 39) and the lack of a control group were important factors limiting our study. Therefore, studies with more patients are needed.
Non-motor symptoms in IPD patients are a major cause of disability and have a significant negative effect on activities of daily living. Fatigue is an important non-motor symptom that is overlooked in clinical follow-up and requires a multidisciplinary approach. With the early recognition and management of fatigue, IPD patients’ adaptation to rehabilitation becomes easier. It also allows the development of individual rehabilitation strategies. Our study will contribute to awareness of non-motor symptoms such as fatigue, which is difficult to treat and manage.
- Friedman JH, Beck JC, Chou KL, Clark G, Fagundes CP, Goetz CG, Herlofson K, Kluger B, Krupp LB, Lang AE, Lou JS, Marsh L, Newbould A, Weintraub D. Fatigue in Parkinson's disease: report from a mutidisciplinary symposium. NPJ Parkinsons Dis. 2016;2:15025–. doi: 10.1038/npjparkd.2015.25. Epub 2016 Jan 14. PMID: 27239558; PMCID: PMC4883681.
- Müller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB. Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson's disease. Parkinsonism Relat Disord. 2013 Nov;19(11):1027-32. doi: 10.1016/j.parkreldis.2013.07.010. Epub 2013 Aug 2. PMID: 23916654.
- Pfeiffer RF. Non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2016 Jan;22 Suppl 1:S119-22. doi: 10.1016/j.parkreldis.2015.09.004. Epub 2015 Sep 3. PMID: 26372623.
- Fabbrini G, Latorre A, Suppa A, Bloise M, Frontoni M, Berardelli A. Fatigue in Parkinson's disease: motor or non-motor symptom? Parkinsonism Relat Disord. 2013 Feb;19(2):148-52. doi: 10.1016/j.parkreldis.2012.10.009. Epub 2012 Oct 26. PMID: 23107555.
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2002 Jan;8(3):193-7. doi: 10.1016/s1353-8020(01)00015-3. PMID: 12039431.
- Karlsen K, Larsen JP, Tandberg E, Jørgensen K. Fatigue in patients with Parkinson's disease. Mov Disord. 1999 Mar;14(2):237-41. doi: 10.1002/1531-8257(199903)14:2<237::aid-mds1006>3.0.co;2-x. PMID: 10091615.
- Miwa H, Miwa T. Fatigue in patients with Parkinson's disease: impact on quality of life. Intern Med. 2011;50(15):1553-8. doi: 10.2169/internalmedicine.50.4954. Epub 2011 Aug 1. PMID: 21804280.
- Friedman J, Friedman H. Fatigue in Parkinson's disease. Neurology. 1993 Oct;43(10):2016-8. doi: 10.1212/wnl.43.10.2016. PMID: 8413960.
- Herlofson K, Ongre SO, Enger LK, Tysnes OB, Larsen JP. Fatigue in early Parkinson's disease. Minor inconvenience or major distress? Eur J Neurol. 2012 Jul;19(7):963-8. doi: 10.1111/j.1468-1331.2012.03663.x. Epub 2012 Feb 16. PMID: 22340430.
- Pont-Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R, Mas N, Hofeneder D, Brücke T, Bayés A, Wenzel K, Infante J, Zach H, Pirker W, Posada IJ, Álvarez R, Ispierto L, De Fàbregues O, Callén A, Palasí A, Aguilar M, Martí MJ, Valldeoriola F, Salamero M, Poewe W, Tolosa E. The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Mov Disord. 2015 Feb;30(2):229-37. doi: 10.1002/mds.26077. Epub 2014 Dec 1. PMID: 25449044.
- Kluger BM, Parra V, Jacobson C, Garvan CW, Rodriguez RL, Fernandez HH, Fogel A, Skoblar BM, Bowers D, Okun MS. The prevalence of fatigue following deep brain stimulation surgery in Parkinson's disease and association with quality of life. Parkinsons Dis. 2012;2012:769506. doi: 10.1155/2012/769506. Epub 2012 May 13. PMID: 22666631; PMCID: PMC3359731.
- Chou KL, Persad CC, Patil PG. Change in fatigue after bilateral subthalamic nucleus deep brain stimulation for Parkinson's disease. Parkinsonism Relat Disord. 2012 Jun;18(5):510-3. doi: 10.1016/j.parkreldis.2012.01.018. Epub 2012 Feb 15. PMID: 22341622.
- Antonini A, Barone P, Marconi R, Morgante L, Zappulla S, Pontieri FE, Ramat S, Ceravolo MG, Meco G, Cicarelli G, Pederzoli M, Manfredi M, Ceravolo R, Mucchiut M, Volpe G, Abbruzzese G, Bottacchi E, Bartolomei L, Ciacci G, Cannas A, Randisi MG, Petrone A, Baratti M, Toni V, Cossu G, Del Dotto P, Bentivoglio AR, Abrignani M, Scala R, Pennisi F, Quatrale R, Gaglio RM, Nicoletti A, Perini M, Avarello T, Pisani A, Scaglioni A, Martinelli PE, Iemolo F, Ferigo L, Simone P, Soliveri P, Troianiello B, Consoli D, Mauro A, Lopiano L, Nastasi G, Colosimo C. The progression of non-motor symptoms in Parkinson's disease and their contribution to motor disability and quality of life. J Neurol. 2012 Dec;259(12):2621-31. doi: 10.1007/s00415-012-6557-8. Epub 2012 Jun 19. PMID: 22711157.
- Stocchi F, Abbruzzese G, Ceravolo R, Cortelli P, D'Amelio M, De Pandis MF, Fabbrini G, Pacchetti C, Pezzoli G, Tessitore A, Canesi M, Iannacone C, Zappia M; FORTE Study Group. Prevalence of fatigue in Parkinson disease and its clinical correlates. Neurology. 2014 Jul 15;83(3):215-20. doi: 10.1212/WNL.0000000000000587. Epub 2014 Jun 13. PMID: 24928125.
- Abe K, Takanashi M, Yanagihara T. Fatigue in patients with Parkinson's disease. Behav Neurol. 2000;12(3):103-6. doi: 10.1155/2000/580683. PMID: 11455047.
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord. 2001 May;16(3):507-10. doi: 10.1002/mds.1099. PMID: 11391746.
- Schifitto G, Friedman JH, Oakes D, Shulman L, Comella CL, Marek K, Fahn S; Parkinson Study Group ELLDOPA Investigators. Fatigue in levodopa-naive subjects with Parkinson disease. Neurology. 2008 Aug 12;71(7):481-5. doi: 10.1212/01.wnl.0000324862.29733.69. PMID: 18695158; PMCID: PMC2937041.
- Bensing JM, Hulsman RL, Schreurs KM. Gender differences in fatigue: biopsychosocial factors relating to fatigue in men and women. Med Care. 1999 Oct;37(10):1078-83. doi: 10.1097/00005650-199910000-00011. PMID: 10524374.
- Brown RG, Dittner A, Findley L, Wessely SC. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005 Jan;11(1):49-55. doi: 10.1016/j.parkreldis.2004.07.007. PMID: 15619463.
- Hagell P, Brundin L. Towards an understanding of fatigue in Parkinson disease. J Neurol Neurosurg Psychiatry. 2009 May;80(5):489-92. doi: 10.1136/jnnp.2008.159772. Epub 2009 Feb 9. PMID: 19204024.