More Information
Submitted: February 08, 2023 | Approved: February 27, 2023 | Published: February 28, 2023
How to cite this article: Green DS, O’Neill D, Dimigen M, Kaur S, Beran RG. Case Report: Contrast Imaging in the Setting of Cerebral Venous Thrombosis. J Neurosci Neurol Disord. 2023; 7: 001-004.
DOI: 10.29328/journal.jnnd.1001072
Copyright License: © 2023 Green DS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: CVST; cerebral; venous; sinus; thrombosis; venogram; contrast; COVID-19
Case Report: Contrast Imaging in the Setting of Cerebral Venous Thrombosis
Daniel Stephen Green1*, Daniel O’Neill2, Marion Dimigen3, Simren Kaur4 and Roy Beran5
1Neurology Registrar, Neurology Department, Liverpool Hospital, Liverpool 2170, NSW, Australia
2Staff Specialist Neurologist, Liverpool Hospital, Liverpool 2170, NSW, Australia
3Staff Specialist Radiologist, Liverpool Hospital, Liverpool 2170, NSW, Australia
4Visiting Radiology Registrar, Prince of Wales Hospital, Randwick 2031, NSW, Australia
5Neurology Department, Liverpool Hospital, Liverpool 2170, NSW, Australia, Ingham Institute of Applied Medical Science, South Western Sydney Local Health District, Conjoint Professor, South Western Clinical School, University of NSW, Sydney, NSW, Australia, Professor, Griffith University, Southport, Qld, Australia, Professor, Sechenov Moscow First State University, Moscow, Russia, Conjoint Professor, Western Sydney University, Sydney, Australia
*Address for Correspondence: Dr. Daniel Stephen Green, Neurology Registrar, Neurology Department, Liverpool Hospital, Liverpool 2170, NSW, Australia, Email: [email protected]
Disruption to contrast agent supply chains for radiology investigations has become an additional consequence of the COVID-19 pandemic. Various recommendations, including dose reductions and choices of alternative agents, have been made to help account for this limited availability. This case demonstrated how two separate computed tomography (CT) venograms with different contrast agents and similar timing, undertaken on the same day for the same patient, yielded different results; cerebral venous thromboses were more prominent in the subsequent scan. Although there were features suggestive of thrombosis on the first scan, repeat imaging was required to further characterize the lesions identified. The case exemplifies the notion that diagnostic imaging should always be guided by a detailed history and examination. It also raises the discussion point of whether more strict or uniform protocols should be developed to facilitate contrast administration for radiology investigations. It is important that appropriate doses are always administered to maximize diagnostic yield.
The COVID-19 pandemic has had profound, multi-faceted effects on the way in which medicine is practiced internationally. One such adverse effect has been the disruption to supply chains of iodinated contrast for CT imaging [1]. Iohexol, sold under the name Omnipaque by GE Healthcare, is one such example. Limited availability has resulted in increased demand for other agents, requiring changes to protocols and requirements for clinicians to rationalize investigations and minimize the utilization of contrast when safe [2]. It appears that the shortage of these agents is likely to continue into the near future.
The American College of Radiology Committee on Drugs and Contrast Media has made recommendations, amongst others, to look for alternative versions of contrast agents and to minimize individual doses administered to reduce waste [3]. These recommendations have also emphasized the importance of not sacrificing image quality by using suboptimal doses. Some institutions, such as the Yale School of Medicine, have adopted dose-reduction techniques of iodinated contrast media as a way to preserve supply [4].
This case describes a patient who had two separate computed tomography (CT) venograms, undertaken on the same day, using different contrast agents with differing concentrations. Although the initial imaging was abnormal and suggestive of possible cerebral venous thrombosis, the second scan was more conclusive. Although other variables, such as volume, affect the degree of contrast administered, appropriate protocols were followed to ensure an optimal imaging result.
A variety of contrast agents and doses are used in clinical practice, but this case provides an important point of reflection to further analyze the efficacy of different contrast agents in the setting of supply issues. In this case, a strong clinical suspicion, informed by a detailed history and exam, prompted the subsequent scan, in the face of a less differentiated initial result, producing a definitive diagnosis.
An 80-year-old male was brought to the emergency department with confusion, reduced level of consciousness, severe headache and vomiting. This was on a background of a ten-day history of right-sided facial pain and two-week history of coryzal symptoms. Approximately one month prior, the patient had undergone surgery for a right jaw abscess. His medical history included diverticular disease, depression, gout and a hiatus hernia. There was no personal history of any malignancies. Regular medications included colchicine, gabapentin and esomeprazole. Prior to this illness, the patient was independent, attended the hospital from home where he lived alone and held important roles in various local community groups. There was no history of excessive alcohol use.
On examination, the patient was drowsy but rousable. He was febrile and hemodynamically stable. There was prominent bilateral proptosis and chemosis with associated complex ophthalmoplegia. Visual acuity was 6/12 in each eye and pupils were equal and reactive to light, at 3 mm diameter. The remainder of the cranial nerve examination was normal. There were no rashes or signs of meningism. Upper limbs demonstrated normal tone, power and coordination. Reflexes were symmetrically brisk in the triceps but otherwise normal in the upper limbs. The lower limb exam demonstrated normal tone, power and coordination. Reflexes were symmetrically brisk at the knees and normal at the ankles. There was no crossed-adductor reflex. Plantar reflexes were down-going. Heart sounds were dual without any murmur and the chest auscultation was clear. The calves were soft and non-tender. There were no peripheral stigmata of endocarditis. Poor dentition was noted. His weight was 65 kg with a height of 160 cm, producing a BMI of 25.4.
Full blood count demonstrated a raised white cell count (22.1 x 10^9/L) with a neutrophilia (19.9 x 10^9/L), low hemoglobin (101 g/L), normal platelets (368 x 10^9/L), raised C-reactive protein (234.4 mg/L), normal creatinine (57 µmol/L) and eGFR 86 mL/min. A 12-lead ECG demonstrated atrial fibrillation. The patient was initiated on empirical treatment for meningoencephalitis. Subsequently, a CT brain and venogram were undertaken, which included assessment of the orbits, and demonstrated complete opacification of the right sphenoid sinus, mild bilateral proptosis and asymmetry of the superior ophthalmic veins. Opacification and assessment of the cavernous sinus was suboptimal. An “Ultravist 300” (iopromide) contrast agent was utilised for this scan. There was no consolidation on the chest x-ray and urine microscopy demonstrated a white cell count of 10-100 x 10^6/L. A lumbar puncture was subsequently undertaken, which revealed a raised cerebrospinal fluid (CSF) red cell count (122 x 10^6/L) and raised white cell count (2117 x 10^6/L), with 1% mononuclear cells and 99% polymorphonuclear cells. CSF protein was elevated (1.23 g/L) and glucose was low (2.4 mmol/L). CSF gram stain was negative.
It remained appropriate to treat for presumed meningoencephalitis. The history of coryzal symptoms and CT features of sinusitis alluded to a possible underlying mechanism. It was difficult to account for the obvious bilateral proptosis with chemosis with the findings from the first CT venogram. TSH was 0.25 mIU/L with normal free T4 (13.1 pmol/L). After discussions between the Neurology team and reporting Radiologist, a recommendation was made to repeat a dedicated CT venogram study. This recommendation was based on the remaining strong clinical suspicion of cavernous sinus thrombosis and concern for whether there may have been underlying technical factors behind the previous imaging acquisition: there was unexplained asymmetry of the superior ophthalmic veins with poor assessment of the cavernous sinuses. A repeat CT venogram was undertaken as magnetic resonance (MR) venography was not readily available. This repeat scan demonstrated occlusive thrombosis of the bilateral superior ophthalmic veins, bilateral cavernous sinuses and right superior petrosal sinus (see Figures 1 to 8 for comparisons between the first and second venogram studies). Non-occlusive thrombosis of the left superior petrosal sinus, right transverse sinus and bilateral internal jugular veins was also identified. An “Omnipaque 350” (iohexol) contrast agent was utilised for this second scan.
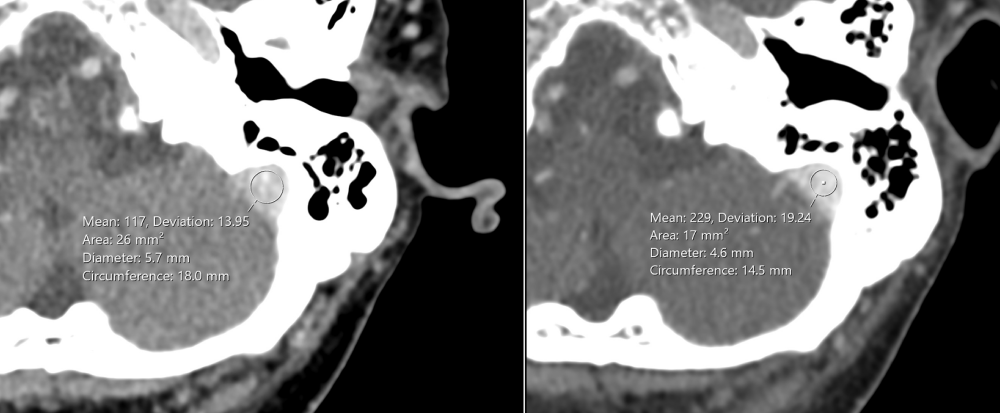
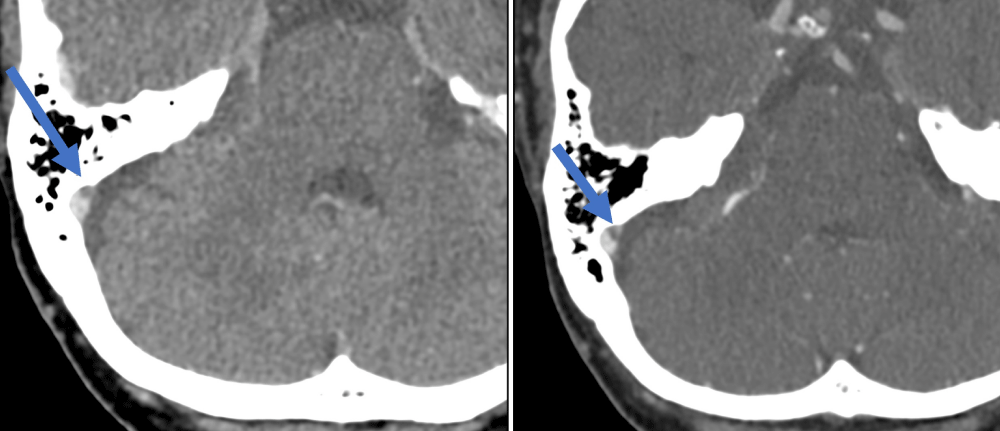
Figure 1: The Hounsfield Unit (HU) density in the second venogram (right image) was higher than in the first venogram (left image) (229 HU versus 117 HU respectively).
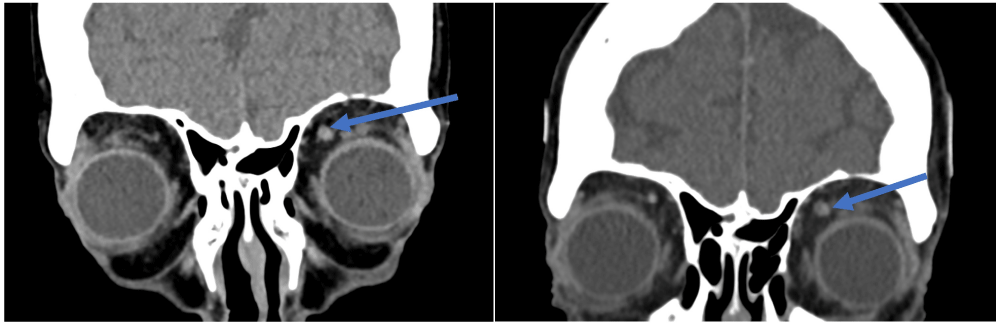
Figure 2: Superior ophthalmic vein thrombus – easier to differentiate on the second venogram (right image) compared to the first venogram (left image).
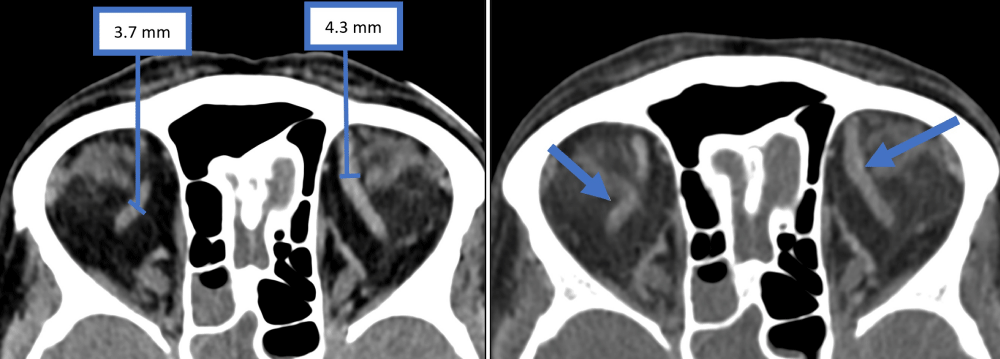
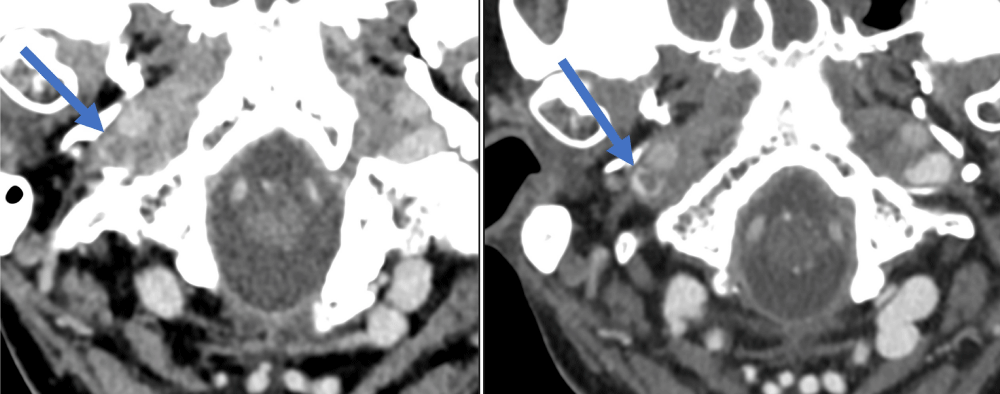
Figure 3: A similar degree of asymmetry in the size of the superior ophthalmic vein was noted in the first venogram (left image) and second venogram (right image).
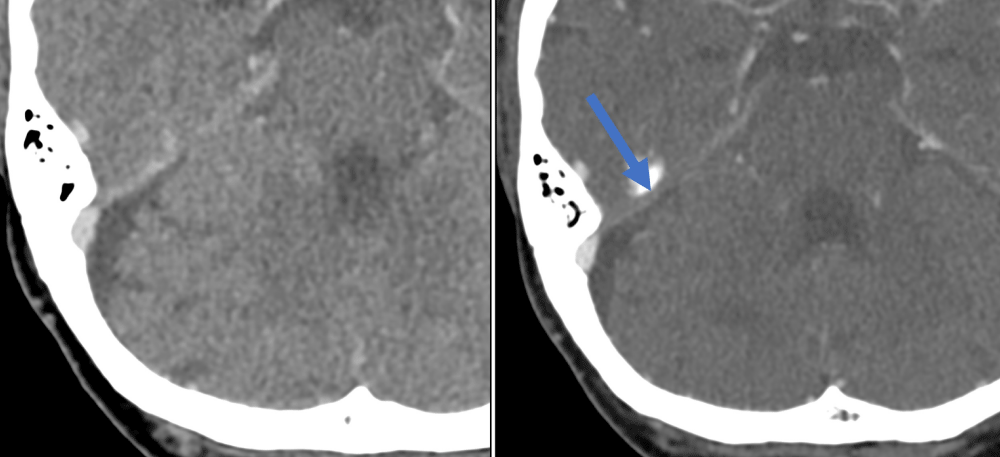
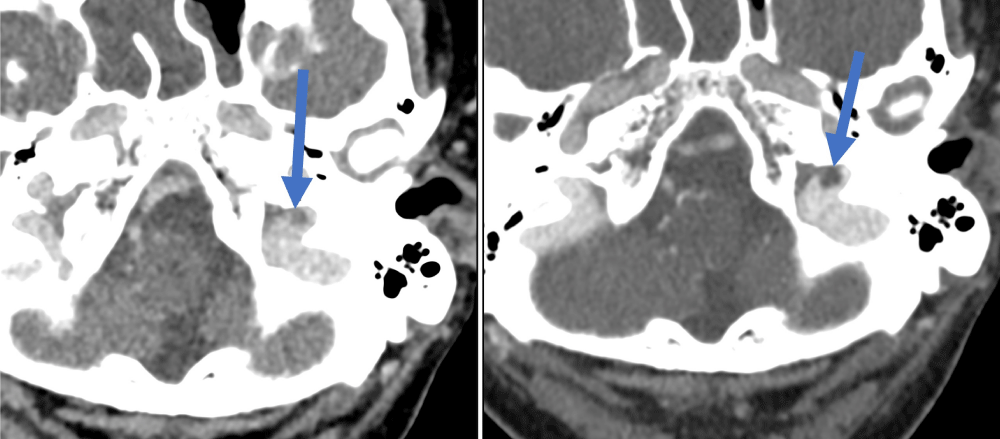
Figure 4: Right superior petrosal sinus thrombus – easier to identify on the second venogram (right image) compared to the first venogram (left image).
Figure 5: Right transverse sinus thrombus – easier to identify on the second venogram (right image) compared to the first venogram (left image).
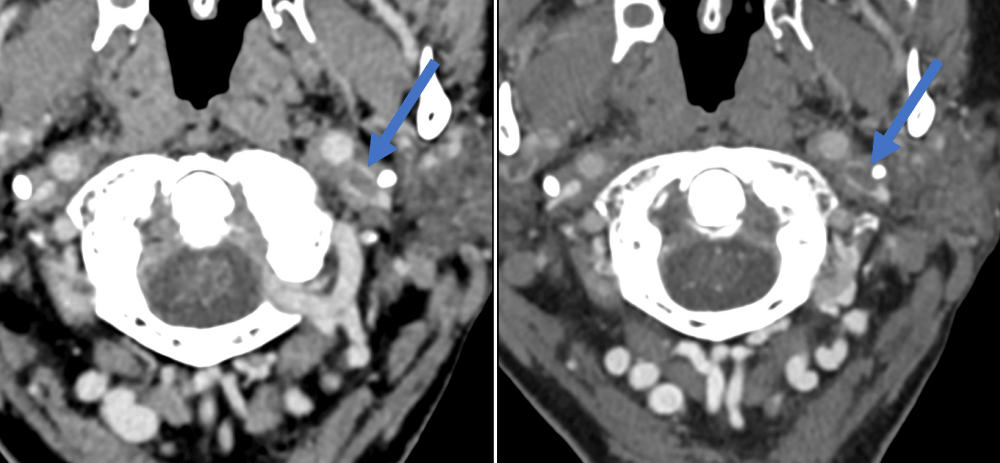
Figure 6: Right internal jugular vein thrombus – easier to identify on the second venogram (right image) compared to first venogram (left image).
Figure 7: Left sigmoid sinus thrombus – easier to identify on the second venogram (right image) compared to the first venogram (left image).
Figure 8: Left internal jugular vein thrombus – easier to identify on the second venogram (right image) compared to the first venogram (left image).
This new result, consistent with the history and clinical syndrome, was suggestive of septic cerebral venous thrombosis, provoked in the setting of complicated sinusitis. A blood culture, taken at the time of admission, later proved positive for Streptococcus intermedius and the urine culture was negative. Ophthalmological consultation excluded an ocular cause for the proptosis. The temporal proximity of this symptom onset to his presentation strongly supported the underlying etiology of a provoked cerebral venous thrombosis.
The patient was treated conservatively for four weeks with intravenous ceftriaxone, following advice from the Infectious Diseases team. Therapeutic anticoagulation treatment was commenced during admission with a heparin infusion. This was subsequently transitioned to dabigatran. The underlying sinusitis was treated with oxymetazoline nasal sprays.
The patient improved following this treatment. The bilateral proptosis and chemosis resolved as did the associated encephalitis. During the admission, there were some difficulties optimizing anticoagulation, with episodes of macroscopic haematuria. The Urology team was consulted and the patient underwent continuous bladder irrigation. A CT IVP demonstrated no suspicious urothelial abnormality. The haematuria resolved and he was discharged with a planned follow-up in the community. The patient recovered well from a mobility and functional perspective and returned to living independently.
This case demonstrated that two CT venograms, under-taken on the same patient, on the same day, yielded different results. Two different iodinated contrast agents were used (“Ultravist 300” (iopromide) for the first scan and “Omnipaque 350” (iohexol) for the second scan). A different contrast agent was utilised for the second scan only due to contrast availability at that particular time. A randomized controlled trial has previously noted similar efficacies between these agents at a concentration of 300 mg I/mL [5]. In the present case, different concentrations of contrast agents were used. Despite this, these concentrations are both technically approved as suitable for venography [6,7]. The radiographers confirmed, at the time of scanning, that both studies were technically acceptable CT venograms despite one being labelled as a post-contrast study. The subsequent CT results directly altered management and improved morbidity. It is imperative to be cognizant of the potential for results not to support clinical diagnosis and to explore this issue further, especially in the setting of the contrast shortages related to the current COVID-19 pandemic.
Clinical features of cerebral venous thrombosis vary and may include a new type of headache, clinical features of raised intracranial pressure, encephalopathy, seizures and non-localizing focal neurological symptoms. There are many factors that may increase the risk of cerebral venous thrombosis, some of which include head trauma, dural arteriovenous malformations, pregnancy, oral contraceptive use, infections, malignancies, vasculitis and other acquired or inherited prothrombotic states [9]. Urgent neuroimaging can establish the diagnosis, with a CT brain and venogram or a magnetic resonance imaging (MRI) brain and venogram [10-12]. Imaging may demonstrate the absence of flow in a venous sinus or cortical vein and/or intraluminal venous thrombus. Various direct signs, such as the empty delta sign, and indirect signs, such as hemorrhagic lesions, may be observed. Cerebral digital subtraction angiography (DSA) is another alternative that can be used if MRI and CT imaging is inconclusive [13]. Although a raised D-dimer may be supportive of a diagnosis of cerebral venous thrombosis, a D-dimer result cannot be used to confirm or exclude the diagnosis [14].
Protocols are in place to minimize contrast wastage but this case serves as a useful caution to ensure that imaging quality is not sacrificed in the process. The fact that two venograms, conducted on the same patient, could yield such different results emphasizes the validity of not relying on post-contrast CT scans to serve as surrogates of formal venogram studies. Dose reduction recommendations can be based on patient weight and other factors, such as imaging equipment [8]. Perhaps there is a need for more streamlined recommendations to facilitate more uniform scanning results.
This case exemplifies the notion that diagnostic imaging should always be guided by a detailed history and examination. Persisting clinical concern regarding a possible cavernous sinus thrombosis prompted a repeat scan, which confirmed the provisional diagnosis and improved the patient’s outcome. Although there was some evidence of thrombosis, on the initial scan, it was inconclusive and required the subsequent second scan.
This case reaffirms that a clinician should not rely solely on medical imaging to reach a diagnosis; imaging should be an adjunct to clinical suspicion. This case highlights the importance of clear communication between clinicians, radiographers and radiologists in optimizing patient outcomes. An MR venogram may have provided further clarity but lack of timely availability translated to an expedited repeat CT venogram being requested. Perhaps additional studies are required to investigate the diagnostic yield of different contrast brands and doses for venogram studies. No comment can be made as to the superiority or inferiority of one contrast agent compared to the other based on these data; there are other variables that may have affected imaging quality for this case.
To protect patient privacy and confidentiality, the patient’s identifying information, including age and sex, were altered.
- Therapeutic Goods Administration; Shortage of iodinated contrast media (contrast) diagnostic agents. 2022. https://www.tga.gov.au/safety/shortages/medicine-shortage-alerts/shortage-iodinated-contrast-media-contrast-diagnostic-agents.
- Bammer R, Amukotuwa SA. Navigating Supply Chain Disruptions of Iodinated Contrast Agent for Neuroimaging and How Business Intelligence Can Help the Decision Process. AJNR Am J Neuroradiol. 2022 Jul;43(7):944-950. doi: 10.3174/ajnr.A7544. Epub 2022 Jun 1. PMID: 35649725; PMCID: PMC9262060.
- American College of Radiology; Statement from the ACR Committee on Drugs and Contrast Media. 2022. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Contrast-Media.
- Cavallo JJ, Pahade JK. Practice Management Strategies for Imaging Facilities Facing an Acute Iodinated Contrast Media Shortage. AJR Am J Roentgenol. 2022 Oct;219(4):666-670. doi: 10.2214/AJR.22.27969. Epub 2022 May 13. PMID: 35549445.
- Goldberg SN, Abrahams J, Drayer BP, Golding S, Bernardino M, Brunetti J. A comparison of iopromide with iopamidol and iohexol for contrast-enhanced computed tomography. Invest Radiol. 1994 May;29 Suppl 1:S76-83; discussion S93. doi: 10.1097/00004424-199405001-00015. PMID: 8071050.
- Therapeutic Goods Administration; ULTRAVIST 300 iopromide 62.34 g/100mL injection bottle (48507) Product Information. 2022. https://www.tga.gov.au/resources/artg/48507.
- US Food and Drug Administration; OMNIPAQUE™ (iohexol) Injection. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018956s099lbl.pdf.
- Kaiser DPO, Abdalkader M, Berberich A, Sporns PB, Nguyen TN. Acute shortage of iodinated contrast media: implications and guidance for neurovascular imaging and intervention. Neuroradiology. 2022 Sep;64(9):1715-1718. doi: 10.1007/s00234-022-02999-6. Epub 2022 Jun 18. PMID: 35716206; PMCID: PMC9206091.
- de Freitas GR, Bogousslavsky J. Risk factors of cerebral vein and sinus thrombosis. Front Neurol Neurosci. 2008;23:23-54. doi: 10.1159/000111259. PMID: 18004052.
- Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I, Masuhr F, Aguiar de Sousa D, Stam J; European Stroke Organization. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur J Neurol. 2017 Oct;24(10):1203-1213. doi: 10.1111/ene.13381. Epub 2017 Aug 20. PMID: 28833980.
- Linn J, Ertl-Wagner B, Seelos KC, Strupp M, Reiser M, Brückmann H, Brüning R. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. AJNR Am J Neuroradiol. 2007 May;28(5):946-52. PMID: 17494676; PMCID: PMC8134361.
- Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Apr;42(4):1158-92. doi: 10.1161/STR.0b013e31820a8364. Epub 2011 Feb 3. PMID: 21293023.
- Sadik JC, Jianu DC, Sadik R, Purcell Y, Novaes N, Saragoussi E, Obadia M, Lecler A, Savatovsky J. Imaging of Cerebral Venous Thrombosis. Life (Basel). 2022 Aug 10;12(8):1215. doi: 10.3390/life12081215. PMID: 36013394; PMCID: PMC9410175.
- Dentali F, Squizzato A, Marchesi C, Bonzini M, Ferro JM, Ageno W. D-dimer testing in the diagnosis of cerebral vein thrombosis: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2012 Apr;10(4):582-9. doi: 10.1111/j.1538-7836.2012.04637.x. PMID: 22257124.