More Information
Submitted: June 15, 2023 | Approved: June 23, 2023 | Published: June 24, 2023
How to cite this article: Acır I, Atay ZOV, Atay M, Yayla V. Sleep Quality and Laboratory Findings in Patients with Varicose Vein Leg Pain. J Neurosci Neurol Disord. 2023; 7: 022-026.
DOI: 10.29328/journal.jnnd.1001077
Copyright License: © 2023 Acır I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Sleep quality; Leg pain; Pittsburgh; laboratory findings
Sleep quality and Laboratory Findings in Patients with Varicose Vein Leg Pain
Ibrahim Acır1* , Zeynep Vildan Okudan Atay1
, Zeynep Vildan Okudan Atay1 , Mehmet Atay2
, Mehmet Atay2 and Vildan Yayla1
and Vildan Yayla1
1Neurology Clinic, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey
2Cardiovascular Surgery Clinic, Bahçelievler Public Hospital, Istanbul, Turkey
*Address for Correspondence: Ibrahim Acır, MD, Neurology Clinic, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey, Email: [email protected]
Aim: This study aimed to investigate the association between sleep quality, as measured by the Pittsburgh Sleep Quality Index (PSQI), and laboratory findings in patients presenting with the complaint of leg pain due to varicose veins.
Materials and Methods: A total of 160 patients with leg pain were included in this study. Sleep quality was assessed using the PSQI, and laboratory tests were conducted to evaluate ferritin, iron, vitamin B12, Thyroid Stimulating Hormone (TSH), C-reactive protein (CRP), albumin, low-density lipoprotein (LDL), and hemoglobin levels. Statistical analyses were performed using the independent t-test or Mann-Whitney U test for continuous variables and the chi-square test for categorical variables.
Results: Patients with poor sleep quality had a significantly higher prevalence of leg pain complaints compared to those with good sleep quality (p < 0.001). Females were more likely to report poor sleep quality (p = 0.006). No significant associations were found between sleep quality and age, smoking status, alcohol use, or pack/year of smoking. Patients with poor sleep quality had significantly lower ferritin levels (p = 0.008), lower albumin levels (p = 0.031), and lower hemoglobin levels (p = 0.036) compared to patients with good sleep quality. However, no significant differences were observed in other laboratory parameters.
Conclusion: The findings suggest a significant association between poor sleep quality and leg pain complaints in patients with varicose veins. Lower ferritin, albumin, and hemoglobin levels in patients with poor sleep quality may indicate potential underlying mechanisms linking sleep quality and leg pain. Addressing sleep quality issues in patients with leg pain could improve overall well-being and treatment outcomes.
Sleep is a fundamental physiological process that plays a vital role in maintaining overall health and well-being. It is an essential time for rest, restoration, and consolidation of various cognitive and physical functions. Disturbances in sleep patterns have been associated with a wide range of physical and mental health conditions, including chronic pain [1-3]. Among the various types of pain, leg pain is a common complaint that can significantly impact an individual’s quality of life. Therefore, understanding the complex relationship between sleep quality and leg pain is of utmost importance in developing effective management and treatment strategies.
Recent studies have suggested that sleep disturbances may be a particular concern for individuals experiencing leg pain associated with varicose veins [4]. Varicose veins are swollen, twisted veins that commonly occur in the legs, causing discomfort, heaviness, and pain [5]. The presence of leg pain can disrupt sleep and lead to further sleep disturbances, creating a cyclical pattern of pain and poor sleep quality. Identifying and addressing these sleep-related issues in individuals with varicose vein leg pain may contribute to improved pain management, overall well-being, and treatment outcomes.
To comprehensively evaluate sleep quality, the PITTSBURGH Sleep Quality Index (PSQI) is widely used as a reliable and validated self-report questionnaire. The PSQI assesses various dimensions of sleep, including sleep duration, disturbances, latency, daytime dysfunction, sleep efficiency, and use of sleep medications [6]. By employing the PSQI, researchers can quantitatively measure sleep quality and evaluate its association with other variables, providing a comprehensive understanding of the sleep profiles of individuals with leg pain due to varicose veins.
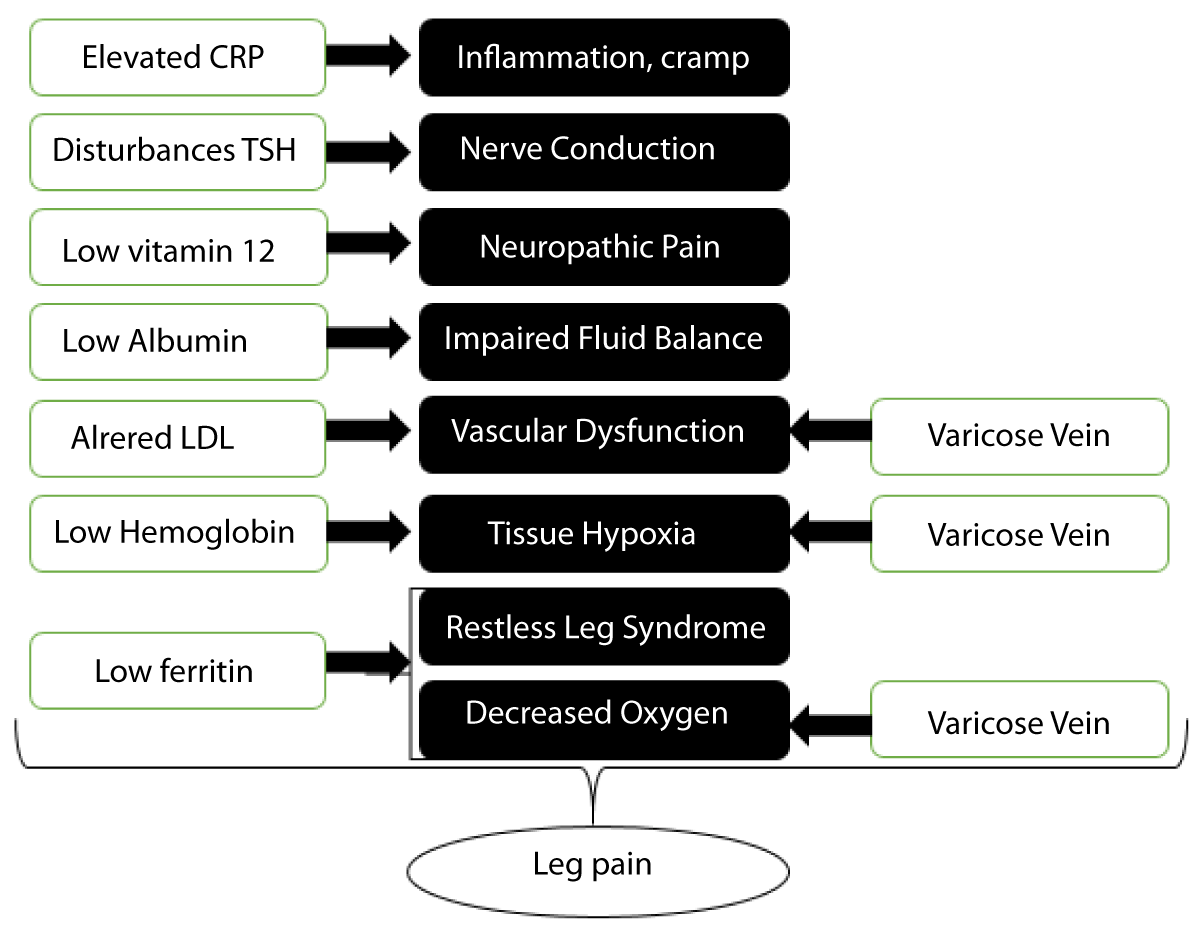
In addition to assessing sleep quality, investigating specific laboratory parameters can provide valuable insights into the potential underlying mechanisms linking sleep quality and leg pain. The selection of these laboratory parameters is based on previous research indicating their relevance to pain perception and the development of chronic pain conditions, particularly in the elderly population [7,8] (Figure 1).
Figure 1: The pathophysiology of leg pain that laboratory parameters and varicose vein can caused.
Ferritin, a protein that stores iron in the body, has been linked to pain modulation and inflammatory processes. Altered ferritin levels may indicate underlying systemic inflammation or iron deficiency, which can contribute to pain symptoms [9]. Iron deficiency can lead to decreased oxygen supply to tissues, potentially exacerbating leg pain in individuals with varicose veins.
Vitamin B12 plays a crucial role in nerve function and the production of red blood cells. Deficiencies in vitamin B12 have been associated with neuropathic pain, which may contribute to the experience of leg pain [10].
Thyroid Stimulating Hormone (TSH) levels reflect thyroid function, and abnormalities in thyroid function have been implicated in pain perception [11]. Thyroid dysfunction can affect various physiological processes, including nerve conduction and pain signaling, potentially influencing leg pain in individuals with varicose veins.
C-reactive protein (CRP) is an inflammatory marker that can indicate the presence of systemic inflammation. Elevated CRP levels have been associated with pain conditions, including chronic pain [12]. Increased inflammation may contribute to the intensity and persistence of leg pain in individuals with varicose veins.
Albumin is an essential protein involved in maintaining fluid balance and transporting various substances in the blood. Altered albumin levels may reflect underlying systemic abnormalities and can potentially impact pain perception and management [13].
Low-density lipoprotein (LDL), often referred to as “bad” cholesterol, has been associated with cardiovascular health. Altered LDL levels may reflect vascular dysfunction and compromised blood flow, which can contribute to leg pain symptoms [14].
Hemoglobin levels reflect the oxygen-carrying capacity of red blood cells. Decreased hemoglobin levels may indicate anemia, which can lead to tissue hypoxia and exacerbate leg pain in individuals with varicose veins [15].
Considering the significant impact of sleep quality on leg pain and the potential role of these laboratory parameters in elucidating underlying mechanisms, this study aims to compare sleep quality levels, as determined by the PSQI, with specific laboratory parameters in patients presenting with leg pain due to varicose veins. Investigating these variables in conjunction can help identify potential associations and provide valuable insights into the complex pathophysiology of leg pain related to sleep quality. By understanding these connections, healthcare professionals can develop targeted interventions and treatment strategies to optimize pain management and enhance the overall well-being of individuals suffering from leg pain associated with varicose veins.
Patients who presented to the Cardiovascular Surgery outpatient clinic with complaints of leg pain and cramps and were diagnosed with venous insufficiency via color Doppler ultrasonography (RDUS) were included in the study. The radiofrequency surgery was used on 160 patients in the study. PSQI test was applied to the patients and other informations were recorded from medical reports.
The PSQI is a 19-item questionnaire that measures various aspects of sleep and provides seven component scores and one composite score. Subjective sleep quality, sleep latency (how long it takes to fall asleep), sleep duration, habitual sleep efficiency (the percentage of time in bed that one is asleep), sleep disturbances, use of sleeping medication, and daytime dysfunction are the component scores.
Each item is weighted on a scale of 0 to 3. The global PSQI score is then computed by adding the seven component scores, yielding an overall score ranging from 0 to 21, with lower scores indicating better sleep quality. 5-21 scores on the PSQI was associated to poor sleep quality.
The demographics and laboratory results of the patients were documented. Patients diagnosed with varicose vein as a result of ultrasound were included in the study. Patients who were under the age of 18, had a history of deep vein thrombosis, had symptomatic peripheral artery disease, or were diagnosed with restless legs were excluded from the study.
Statistical analysis was performed using IBM SPSS Statistics for Windows 20.0. Data distribution was assessed using the Shapiro-Wilk test. Numerical variables were presented as mean ± standard deviation or median (min-max) for normally and non-normally distributed data, respectively. Categorical variables were expressed as numbers and percentages. The Student’s T test or Mann-Whitney U test was used to compare numerical variables between groups, while the Chi-Square test or Fisher’s exact test was used to analyze the relationship between categorical variables. Changes in numerical measurements after the operation were evaluated using the Wilcoxon test, and categorical variables were assessed using McNemar or marginal homogeneity tests. A significance level of P < 0.05 was considered statistically significant.
Written consent was obtained from the patients. Ethics committee approval was obtained.
The results of this study showed that patients with poor sleep quality had a significantly higher prevalence of leg pain complaints compared to those with good sleep quality (p < 0.001). Among the demographic variables, gender was significantly associated with sleep quality, with a higher proportion of females reporting poor sleep quality (p = 0.006) (Table 1). However, no significant association was found between sleep quality and age, smoking status, alcohol use, or pack/year of smoking.
| Table 1: Sleep quality and demographic parameters | |||
| Variables | Sleep Quality | p | |
| Good | Poor | ||
| Age, years | 50,4 ± 11,6 | 47,9 ± 10,0 | 0,164 |
| Gender, n(%) | |||
| Female | 26 (52,0) | 83 (75,5) | 0,006* |
| Male | 24 (48,0) | 27 (24,5) | |
| Smoker, n(%) | 13 (26,0) | 25 (22,7) | 0,691 |
| Pack/year | 8 (1-30) | 13 (2-30) | 0,11 |
| Alcohol user, n(%) | 1 (2,0) | 1 (0,9) | 0,999 |
| Leg Pain, n(%) | 3(6,0) | 87 (79,1) | 0,001* |
| Data were shown as mean ± SD or median (min-max) or numbers and percentage. | |||
Regarding laboratory findings, patients with poor sleep quality had significantly lower ferritin levels (p = 0.008), lower albumin levels (p = 0.031), and lower hemoglobin levels (p = 0.036) compared to patients with good sleep quality. However, no significant differences were observed in iron, B12, TSH between the two groups (Table 2).
| Table 2: Sleep quality and Laboratory parameters. | |||
| Laboratory Values | Sleep Quality | p | |
| Good | Poor | ||
| Ferritin (ng/mL) | 74,1 (4,3-421) | 46,9 (5,2-302) | 0,008* |
| Low (< 12 ng/mL) | 4 (8,0) | 18 (16,4) | 0,239 |
| Iron (mcg/dL) | 80 (24-192) | 74 (15-201) | 0,206 |
| Vitamin B12 (pmol/L) | 285 (113-1000) | 308 (62-2000) | 0,635 |
| TSH (mIU/L) | 1,6 (0,3-28,6) | 1,6 (0,3-57,1) | 0,985 |
| CRP (mg/L) | 1,7 (0,5-16,2) | 2,1 (0,2-26,4) | 0,148 |
| Albumin (g/dL) | 45,8 ± 2,0 | 44,0 ± 6,7 | 0,031* |
| LDL (mg/dL) | 113 (54-196) | 112,5 (41-253) | 0,608 |
| Hemoglobin (g/dl) | 14,1 ± 1,8 | 13,6 ± 1,4 | 0,036* |
| Data were shown as mean ± SD or median (min-max) or numbers and percentages. * p < 0.05 indicates statistical significance. | |||
The findings from our study shed light on the relationship between sleep quality and various demographic, lifestyle factors, and laboratory values. Understanding these associations is crucial for developing targeted interventions to improve sleep quality and overall well-being. It is important to note that the patients included in this study were individuals experiencing leg pain associated with varicose veins, which may have influenced their sleep quality and the observed associations.
Age is often considered a potential factor influencing sleep quality [5]. However, our study did not find a significant association between age and sleep quality in this specific patient population. This suggests that age may not be a strong predictor of sleep disturbances in individuals with varicose vein-related leg pain. It is worth noting that our study included a relatively narrow age range, and further research with a more diverse age distribution among varicose vein patients may be warranted to explore potential age-related effects on sleep quality within this specific context.
Gender emerged as a significant predictor of sleep quality among patients with varicose vein-related leg pain. The discrepancy between our study and existing literature, which found poor sleep quality more prevalent in females compared to males, raises intriguing questions [16]. It is possible that the discrepancy between our findings and the existing literature can be attributed to differences in sample characteristics, cultural factors, or specific study design. For instance, our study may have included a larger proportion of females who reported leg pain due to varicose veins, which could have contributed to the observed gender difference in sleep quality. Moreover, hormonal fluctuations during the menstrual cycle and pregnancy in females can influence sleep patterns and quality [17-20]. Such as estrogen and progesterone levels, as well as socio-cultural factors and differential responses to pain and discomfort, may contribute to this gender difference in sleep quality among individuals with varicose veins [21].
Smoking status and alcohol use are lifestyle factors that have been associated with sleep disturbances in previous studies [22,23]. However, our results did not reveal a significant association between smoking status or alcohol use and sleep quality in this specific patient population. These findings suggest that other factors, such as the direct impact of varicose veins and leg pain on sleep quality, may overshadow the potential effects of smoking and alcohol. It is worth noting that smoking and alcohol use can have detrimental effects on circulatory health, which may exacerbate varicose vein symptoms and indirectly impact sleep quality [24,25]. Future research should consider exploring these complex interactions further.
Laboratory values, such as ferritin and albumin levels, showed significant associations with sleep quality among patients with varicose vein-related leg pain [26]. The lower ferritin and albumin levels observed in individuals with poorer sleep quality highlight the potential impact of varicose veins on these laboratory values. Varicose veins can lead to impaired circulation, affecting nutrient and oxygen supply to the surrounding tissues. As a result, ferritin levels, which reflect iron storage, and albumin levels, an indicator of protein status, may be influenced. The compromised circulation and potential chronic inflammation associated with varicose veins can contribute to decreased ferritin and albumin levels [27-29]. Understanding these associations is important as it suggests that addressing sleep quality issues in individuals with varicose vein-related leg pain may not only alleviate pain symptoms but also potentially improve iron and protein status, thereby positively impacting overall well-being. Lower ferritin levels have been linked to restless leg syndrome and periodic limb movements during sleep, which can disrupt sleep quality [30]. Similarly, albumin plays a role in regulating fluid balance, and alterations in its levels may affect sleep quality through changes in fluid dynamics. These findings highlight the potential utility of ferritin and albumin levels as biomarkers for assessing sleep quality in patients with varicose veins and leg pain [29,31].
It is crucial to acknowledge some limitations of our study within the context of varicose vein patients. First, the lack of a control group in our study was a shortcoming. Second, our study relied on self-report measures for assessing sleep quality, which may be subject to biases and inaccuracies. Lastly, our sample size was relatively small, which may have influenced the statistical power to detect significant associations, particularly in the case of less prevalent variables within this specific patient population.
In conclusion, our study contributes to the existing body of knowledge on sleep quality among patients with varicose veins and leg pain by exploring the relationships between sleep quality and various demographic, lifestyle factors, and laboratory values. While age, smoking status, and alcohol use did not show significant associations with sleep quality within this specific context, gender, ferritin levels, and albumin levels emerged as significant predictors. These findings have implications for personalized interventions targeting sleep disturbances in patients with varicose veins, highlighting the importance of considering individual differences, the specific context of varicose veins and leg pain, and physiological markers when assessing and improving sleep quality. Further research incorporating larger sample sizes, objective sleep measures, and longitudinal designs specifically within the varicose vein patient population is necessary to corroborate our findings and advance our understanding of the complex interplay between sleep quality and various factors in this context.
- Tatlı SZ, Şentürk Cankorur V. Innovations in Depression Information and Treatment – New Developments in Depression with Cases: Approach to Cognitive Disorders in Depression. Current Approach in Psychiatry. 2022; 12(3): 315-332.
- Can KC, Tatlı SZ, Devrimci Özgüven H. Depression in pregnancy and postpartum period. Devrimci Ozguven, H., Editor. Depression. 1st Edition. Ankara: Türkiye Clinics. 2021; 64-74.
- Can SS, Tatlı SZ. Major and Minor Neurocognitive Disorders: Dementia with Lewy Bodies. Turkey Clinics J Psychiatry-Special Topics. 2017; 10(1): 28-36.
- Marshansky S, Mayer P, Rizzo D, Baltzan M, Denis R, Lavigne GJ. Sleep, chronic pain, and opioid risk for apnea. Prog Neuropsychopharmacol Biol Psychiatry. 2018 Dec 20;87(Pt B):234-244. doi: 10.1016/j.pnpbp.2017.07.014. Epub 2017 Jul 19. PMID: 28734941.
- Pieper B, Templin TN. Sleep Quality: A Pilot Study Comparing Patients With and Without Injection-Related Venous Ulcers. J Wound Ostomy Continence Nurs. 2016 Sep-Oct;43(5):471-6. doi: 10.1097/WON.0000000000000254. PMID: 27488737.
- Hartmann JA, Carney CE, Lachowski A, Edinger JD. Exploring the construct of subjective sleep quality in patients with insomnia. J Clin Psychiatry. 2015 Jun;76(6):e768-73. doi: 10.4088/JCP.14m09066. PMID: 26132684.
- Dagnino APA, Campos MM. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front Hum Neurosci. 2022 Mar 3;16:736688. doi: 10.3389/fnhum.2022.736688. PMID: 35308613; PMCID: PMC8928105.
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4. PMID: 2748771.
- Motonishi S, Tanaka K, Ozawa T. Iron deficiency associates with deterioration in several symptoms independently from hemoglobin level among chronic hemodialysis patients. PLoS One. 2018 Aug 2;13(8):e0201662. doi: 10.1371/journal.pone.0201662. Erratum in: PLoS One. 2018 Sep 24;13(9):e0204789. PMID: 30071093; PMCID: PMC6072073.
- Julian T, Syeed R, Glascow N, Angelopoulou E, Zis P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients. 2020 Jul 25;12(8):2221. doi: 10.3390/nu12082221. PMID: 32722436; PMCID: PMC7468922.
- Nishioka K, Uchida T, Usui C, Tanaka R, Matsushima T, Matsumoto Y, Nakamura I, Nishioka K, Hattori N. High prevalence of anti-TSH receptor antibody in fibromyalgia syndrome. Int J Rheum Dis. 2017 Jun;20(6):685-690. doi: 10.1111/1756-185X.12964. Epub 2016 Nov 30. PMID: 27905199.
- Calapai F, Mondello E, Mannucci C, Sorbara EE, Gangemi S, Quattrone D, Calapai G, Cardia L. Pain Biomarkers in Cancer: An Overview. Curr Pharm Des. 2021;27(2):293-304. doi: 10.2174/1381612826666201102103520. PMID: 33138755.
- Hayashi T, Ikehata S, Matsuzaki H, Yasuda K, Makihara T, Futamura A, Arakawa Y, Kuki R, Fukuura K, Takahashi H, Mori N, Higashiguchi T, Yamadaa S. Influence of serum albumin levels during opioid rotation from morphine or oxycodone to fentanyl for cancer pain. Biol Pharm Bull. 2014;37(12):1860-5. doi: 10.1248/bpb.b14-00119. PMID: 25590058.
- Kumagai G, Wada K, Tanaka T, Kudo H, Asari T, Chiba D, Ota S, Nakaji S, Ishibashi Y. Associations between neck symptoms and LDL cholesterol in a cross-sectional population-based study. J Orthop Sci. 2018 Mar;23(2):277-281. doi: 10.1016/j.jos.2017.11.002. Epub 2017 Nov 22. PMID: 29174032.
- Fukushima T, Nakano J, Ishii S, Natsuzako A, Kawachi H, Sakamoto J, Miyazaki Y, Okita M. Influence of Hemoglobin Level on Muscle and Physical Functions, Activities of Daily Living, and Quality of Life in Patients With Hematological Malignancies. Integr Cancer Ther. 2019 Jan-Dec;18:1534735419842196. doi: 10.1177/1534735419842196. PMID: 30947558; PMCID: PMC6452594.
- Gupta R, Ulfberg J, Allen RP, Goel D. Comparison of Subjective Sleep Quality of Long-Term Residents at Low and High Altitudes: SARAHA Study. J Clin Sleep Med. 2018 Jan 15;14(1):15-21. doi: 10.5664/jcsm.6870. PMID: 29198293; PMCID: PMC5734886.
- Pengo MF, Won CH, Bourjeily G. Sleep in Women Across the Life Span. Chest. 2018 Jul;154(1):196-206. doi: 10.1016/j.chest.2018.04.005. Epub 2018 Apr 19. PMID: 29679598; PMCID: PMC6045782.
- Frange C, Franco AM, Brasil E, Hirata RP, Lino JA, Mortari DM, Ykeda DS, Leocádio-Miguel MA, D'Aurea CVR, Silva LOE, Telles SCL, Furlan SF, Peruchi BB, Leite CF, Yagihara FT, Campos LD, Ulhôa MA, Cruz MGDR, Beidacki R, Santos RB, de Queiroz SS, Barreto S, Piccin VS, Coelho FMS, Studart L, Assis M, Drager LF. Practice recommendations for the role of physiotherapy in the management of sleep disorders: the 2022 Brazilian Sleep Association Guidelines. Sleep Sci. 2022 Oct-Dec;15(4):515-573. doi: 10.5935/1984-0063.20220083. PMID: 36419815; PMCID: PMC9670776.
- Reid KJ, Facco FL, Grobman WA, Parker CB, Herbas M, Hunter S, Silver RM, Basner RC, Saade GR, Pien GW, Manchanda S, Louis JM, Nhan-Chang CL, Chung JH, Wing DA, Simhan HN, Haas DM, Iams J, Parry S, Zee PC. Sleep During Pregnancy: The nuMoM2b Pregnancy and Sleep Duration and Continuity Study. Sleep. 2017 May 1;40(5):zsx045. doi: 10.1093/sleep/zsx045. PMID: 28369543; PMCID: PMC6396817.
- Wilson DL, Fung AM, Pell G, Skrzypek H, Barnes M, Bourjeily G, Walker SP, Howard ME. Polysomnographic analysis of maternal sleep position and its relationship to pregnancy complications and sleep-disordered breathing. Sleep. 2022 Apr 11;45(4):zsac032. doi: 10.1093/sleep/zsac032. PMID: 35150285; PMCID: PMC8996027.
- Vincent K, Tracey I. Hormones and their Interaction with the Pain Experience. Rev Pain. 2008 Dec;2(2):20-4. doi: 10.1177/204946370800200206. PMID: 26526773; PMCID: PMC4589942.
- Zheng D, Yuan X, Ma C, Liu Y, VanEvery H, Sun Y, Wu S, Gao X. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021 Oct;24(15):4851-4858. doi: 10.1017/S1368980020004553. Epub 2020 Nov 13. PMID: 33183388.
- Parrino L, Halasz P, Szucs A, Thomas RJ, Azzi N, Rausa F, Pizzarotti S, Zilioli A, Misirocchi F, Mutti C. Sleep medicine: Practice, challenges and new frontiers. Front Neurol. 2022 Oct 14;13:966659. doi: 10.3389/fneur.2022.966659. PMID: 36313516; PMCID: PMC9616008.
- Purani H, Friedrichsen S, Allen AM. Sleep quality in cigarette smokers: Associations with smoking-related outcomes and exercise. Addict Behav. 2019 Mar;90:71-76. doi: 10.1016/j.addbeh.2018.10.023. Epub 2018 Oct 17. PMID: 30368021; PMCID: PMC6324958.
- Colrain IM, Nicholas CL, Baker FC. Alcohol and the sleeping brain. Handb Clin Neurol. 2014;125:415-31. doi: 10.1016/B978-0-444-62619-6.00024-0. PMID: 25307588; PMCID: PMC5821259.
- Razeghi E, Sahraian MA, Heidari R, Bagherzadeh M. Association of inflammatory biomarkers with sleep disorders in hemodialysis patients. Acta Neurol Belg. 2012 Mar;112(1):45-9. doi: 10.1007/s13760-012-0003-7. Epub 2012 Feb 2. PMID: 22427289.
- Christopoulos D, Nicolaides AN, Szendro G. Venous reflux: quantification and correlation with the clinical severity of chronic venous disease. Br J Surg. 1988 Apr;75(4):352-6. doi: 10.1002/bjs.1800750419. PMID: 3359149.
- Zamboni P, Scapoli G, Lanzara V, Izzo M, Fortini P, Legnaro R, Palazzo A, Tognazzo S, Gemmati D. Serum iron and matrix metalloproteinase-9 variations in limbs affected by chronic venous disease and venous leg ulcers. Dermatol Surg. 2005 Jun;31(6):644-9; discussion 649. doi: 10.1111/j.1524-4725.2005.31611. PMID: 15996413.
- Zamboni P, Izzo M, Tognazzo S, Carandina S, De Palma M, Catozzi L, Caggiati A, Scapoli G, Gemmati D. The overlapping of local iron overload and HFE mutation in venous leg ulcer pathogenesis. Free Radic Biol Med. 2006 May 15;40(10):1869-73. doi: 10.1016/j.freeradbiomed.2006.01.026. Epub 2006 Feb 14. PMID: 16678024.
- Benediktsdottir B, Janson C, Lindberg E, Arnardóttir ES, Olafsson I, Cook E, Thorarinsdottir EH, Gislason T. Prevalence of restless legs syndrome among adults in Iceland and Sweden: Lung function, comorbidity, ferritin, biomarkers and quality of life. Sleep Med. 2010 Dec;11(10):1043-8. doi: 10.1016/j.sleep.2010.08.006. Epub 2010 Oct 18. PMID: 20961808.
- Kim KM, Hwang HR, Kim YJ, Lee JG, Yi YH, Tak YJ, Lee SH, Chung SI. Association between Serum-Ferritin Levels and Sleep Duration, Stress, Depression, and Suicidal Ideation in Older Koreans: Fifth Korea National Health and Nutrition Examination Survey 2010-2012. Korean J Fam Med. 2019 Nov;40(6):380-387. doi: 10.4082/kjfm.18.0097. Epub 2019 Nov 20. PMID: 31779065; PMCID: PMC6887761.