More Information
Submitted: February 01, 2024 | Approved: February 15, 2024 | Published: February 16, 2024
How to cite this article: Alalykina ES, Sergeeva TN, Ananyan MA, Cherenkov IA, Sergeev VG. A Water-soluble Form of Dihydroquercetin Reduces LPS-induced Astrogliosis, Vascular Remodeling, and mRNA VEGF-A Levels in the Substantia Nigra of Aged Rats. J Neurosci Neurol Disord. 2024; 8: 014-019.
DOI: 10.29328/journal.jnnd.1001092
Copyright License: © 2024 Alalykina ES, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Neuroinflammation; Astroglia; Microvasculature; Ageing; Water-soluble form of dihydroquercetin
A Water-soluble Form of Dihydroquercetin Reduces LPS-induced Astrogliosis, Vascular Remodeling, and mRNA VEGF-A Levels in the Substantia Nigra of Aged Rats
Elena S Alalykina1, Tatyana N Sergeeva1, Michail A Ananyan2*, Ivan A Cherenkov1 and Valeriy G Sergeev1,3
1Department of Physiology, Cell Biology and Biotechnology, Udmurt State University, Izhevsk, Russia
2Advanced Technologies Ltd., Moscow, Russia
3Research Laboratory, Izhevsk State Medical Academia, Izhevsk, Russia
*Address for Correspondence: Michail A Ananyan, Advanced Technologies Ltd., Moscow, Russia, Email: [email protected]
Background: The age-dependent sporadic form of PD is characterized by the degeneration of dopaminergic (DA) neurons in the Substantia Nigra (SN), gliosis, and vascular changes. Vascular changes may contribute to the onset of the disease and exacerbate the neurodegenerative process, as some vascular changes occur before the onset of neuronal loss. To demonstrate the anti-neuroinflammatory efficacy of a new compound, a water-soluble form of dihydroquercetin (DHQ-WF), we studied the structural changes of microcirculatory vasculature, astroglial GFAP, and vascular endothelial growth factor –A (VEGF-A) mRNA expression in the SN of young and old rats after unilateral nigral treatment by lipopolysaccharide (LPS) and oral administration of DHQ-WF.
Materials and methods: The experiments were performed on 18 young (8 weeks - 10 weeks old; 250 g - 320 g) and 18 old (18 months - 19 months old; 390 g - 450 g) male Vistar rats. Young and adult rats from the experimental groups were stereotactically injected with 2 μL LPS solution (LPS from Escherichia coli; 0,01 μL/mL) into one side of the SN. Control young and old rats were similarly injected with 2 μL sterile saline. Half of the animals in both the control and experimental groups (6 animals in each group) received a 2 ml solution containing DHQ-WF at a concentration of 3 mg/ml orally every day. After 8 weeks, brains were harvested and serial cryostat sections were prepared for histochemical (FITC-labeled tomato lectin), immunohistochemical (anti-GFAP Antibody, Cy3 Conjugate) staining, and real-time PCR (mRNA VEGF-A).
Results: Eight weeks after LPS injection into the SN, a significant excess of areas occupied by cell bodies and processes of astroglial cells, the density of microcirculatory vessels, and mRNA VEGF-A expression was observed in old animals compared to control old animals and young LPS-treated rats. Oral administration of DHQ-WF to LPS-treated rats resulted in a significant reduction of these parameters in old animals.
Conclusion: Injection of LPS into rat SN induces neuroinflammation and vascular angiogenesis, maximally expressed in old animals. Administration of DHQ-WF for 8 weeks significantly reduces these LPS-induced changes. DHQ-WF may be an effective treatment for reducing the effects of neuroinflammation in the aging brain.
Aging is a major risk factor for several neurodegenerative diseases [1], including Parkinson’s disease (PD) [2], which is characterized by progressive degeneration of dopaminergic (DA) neurons the in the substantia nigra compacta (SNc) [3]. The neurodegenerative process is preceded by chronic neuroinflammation characterized by gliosis [4] as well as vascular changes, disruption of the Blood-Brain Barrier (BBB), and abnormalities in cerebral blood flow [5,6]. Vascular alterations can contribute to disease onset and aggravate the neurodegenerative process as some vascular changes already occur before the onset of neuronal loss or behavioral deficits in animal models of the respective disease [7,8]. Previous studies have reported changes in SN vascularization in PD patients and animal models of PD [9,10]. Similarly, aging is known to cause changes in angiogenesis in the brain and other tissues [11].
Vascular Endothelial Growth Factor (VEGF) is a key mediator of angiogenesis and vascular permeability [12,13], and is thought to be involved in aging-induced vascular changes observed in PD and PD animal models [10,14].
In recent years, researchers have increasingly focused on the potential use of plant polyphenolic secondary metabolites as antioxidant and anti-inflammatory agents, particularly taxifolin (3, 5, 7, 3, 3, and 4-pentahydroxy flavanone), also known as Dihydroquercetin (DHQ). DHQ has been confirmed to exhibit various pharmacological activities, including anti-inflammation and regulation of oxidative stress effects [15]. An in vitro study has shown that taxifolin is able to inhibit LPS-induced activation of the mRNA and protein levels of inducible nitric oxide synthase (iNOS) and VEGF [16]. The water-soluble form of dihydroquercetin (DHQ-WF) demonstrated an even more pronounced neuroprotective effect under in vitro conditions [17].
However, there is no data on the protective properties of the DHQ-WF and its mechanisms of action in PD models in animals of different ages. In this study, we examined VEGF expression, vascular remodeling, and astroglia activation in the SN of young and old rats in response to stereotactic injection of lipopolysaccharide (LPS) and subsequent oral administration of DHQ-WF.
Experimental design
Experiments were performed on 18 young (8 weeks - 10 weeks old; 250 g - 320 g) and 18 old (18 months - 19 months old; 390 g - 450 g) Vistar male rats. All animal procedures were performed in accordance with the Rules of Laboratory Practice in Russia (Order No. 267 of the Ministry of Health of the Russian Federation; 19 June 2003). The experimental protocol fulfilled the requirements of the EU Directive 2010/63/EU and was approved by the Bioethics Committees of Izhevsk State Medical Academy (Protocol № 634, 11.12.2008). Using a stereotaxic device, we injected the 2 µl LPS solution (LPS from Escherichia coli O55:B5, Sigma-Aldrich, USA, L2880; 0.01 µl/ml) into the SN of young (n = 12) and old (n = 12) rats (experimental groups) and 2 µl of sterile saline into the SN of control young (n = 6) and old (n = 6) rats. Half of the animals from the experimental groups (consisting of 6 animals from each age group) were given 2 ml of DHQ-VF solution (Advanced Technologies Ltd., Russia) 3 mg/ml orally daily. After 8 weeks, animals were euthanized, brains were sampled and serial cryostat sections (14 µm) were obtained for histochemical (FITC-labelled tomato lectin) and immunohistochemical (anti-GFAP Antibody, Cy3 conjugate) staining, and real-time PCR analysis of SN-containing fragments isolated from a ventral midbrain section on the LPS administration side (mRNA VEGF-A).
Histochemistry and immunohistochemistry
Histochemical staining of vascular endothelium was performed on frozen sections incubated with FITC-conjugated Lycopersicon esculentum (tomato) lectin (1:500, Sigma-Aldrich, USA; L0401).
Immunohistochemical staining was performed using antibodies to GFAP conjugated to Cy3 (1: 500, Sigma-Aldrich, USA, MAB3402C3). The study was performed in a Nikon Eclipse E200 fluorescent microscope equipped with a digital camera MicroPublisher 3.3. Isolectin-positive vessels and immunopositive cell bodies and processes were quantified in 8 standard rectangular regions (620 x 480 mm) encompassing the SN of at least six brain sections per animal. Digitized images were enhanced for contrast to differentiate positive structures from background, and a thresholding scheme was implemented to measure immunoreactive areas as a percentage of the standard rectangular area using Image-Pro Insight 8.0 (Media Cybernetics, USA). Microvessels not larger than 10 µm in diameter, including capillaries and venules, were specifically selected for morphometric analysis. Quantitative analysis of vascular remodeling was performed using AngioTool [18].
RNA extraction and real-time quantitative reverse transcriptase PCR
Total RNA from the ventral midbrain slices was extracted with Trizol (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. Total RNA (2 mg) was reverse transcribed to cDNA with deoxyribonucleotide triphosphates, random primers, and Moloney murine leukemia virus reverse transcriptase (200 U; Invitrogen). Real-time PCR was used to examine relative levels of VEGF-A mRNA. VEGF-A primers sequences were: forward GCACTGGACCCTGGCTTTAC, reverse CCACCAGGGTCTCAATCGGA. b-Actin was used to normalize the number of cDNA samples (forward TCGTG CGTGACATTAAAGAG, reverse TGCCACAGGATTCC ATACC). Experiments were performed with a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The comparative Ct (threshold cycle) method was used to examine the relative mRNA expression
Statistical analysis
Statistical analysis was performed using Statistica 10.0 software (SPSS Inc., Chicago, IL, USA). Data were expressed as a percentage relative to young control, the arithmetic mean of which was taken as 100%. Result distributions were tested for normality using the Shapiro–Wilk W test. One-way analysis of variance (ANOVA) with Tukey’s post-hoc test for multiple comparisons between study groups. Results were considered statistically significant when p < 0.05.
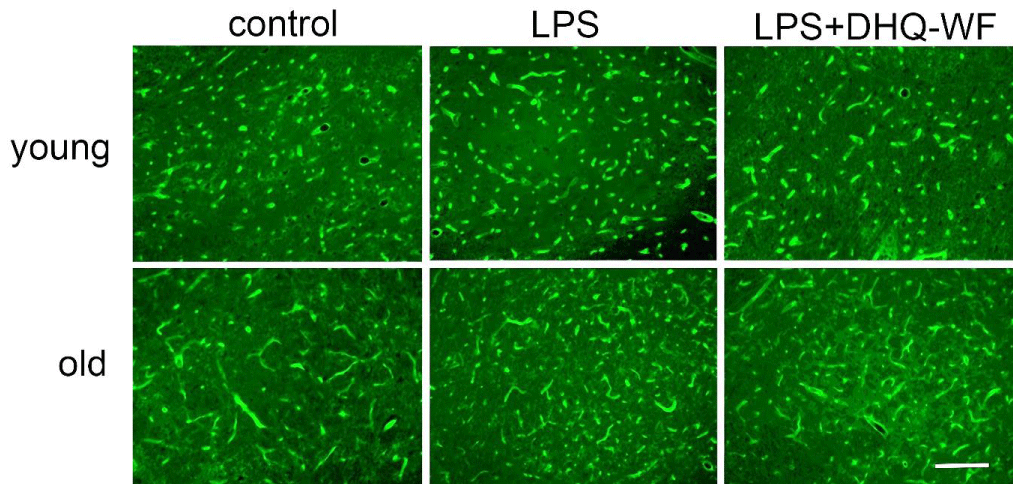
Comparative analysis of microvessel morphology labeled with tomato lectin (Figure 1) and VEGF mRNA expression in the SN of rats under normal and experimental conditions showed significant differences between animals of different ages.
Figure 1: Photomicrographs of nigral microvessels from young and aged rats marked with FITC-conjugated tomato lectin 8 weeks after administration of sterile saline (control), lipopolysaccharide (LPS), and lipopolysaccharide with simultaneous oral administration of a water-soluble form of dihydroquercetin (LPS+DHQ-WF). Line length = 100 µm.
Thus, the mean vessel length in old control animals, counted on a standardized plot, exceeded this index in young individuals of the control group by 77.6% (p < 0.05) (Table 1), but the number of microvessels was lower by 22.6% (p < 0.05). Eight weeks after a single injection of LPS into the SN, young rats showed a slight trend toward an increase in mean vessel length in this region compared with the corresponding control. At the same time, significant vascular changes were observed in old animals after intranigral injection of LPS. There was a significant 87.3% (p < 0.001) increase in vessel number and by 32.8% (p < 0.05) decrease in mean vessel length compared to age-matched control animals.
| Table 1: VEGF mRNA expression and morphometric parameters of nigral vessels and astroglia in control and experimental young and old rats. | ||||||
| Morphometric parameters and mRNA expression |
Age | Investigated groups of rats | p-value LPS/ control |
p-value LPS/ LPS+DHQ-WF |
||
| Control | LPS | LPS+DHQ-WF | ||||
| Vessel length | Young | 100.0 ± 18.8 | 110.5 ± 12.6 | 112.7 ± 13.6 | 0.58 | 0.46 |
| Old | 177.6 ± 23.6 | 144.9 ± 19.0 | 133.0 ± 18.3 | 0.026 | 0.59 | |
| Number of vessels | Young | 100.0 ± 18.8 | 116.5 ± 12.6 | 112.7 ± 13.6 | 0.58 | 0.46 |
| Old | 77.6 ± 13.6 | 164.9 ± 17.0 | 139.0 ± 18.3 | 0.026 | 0.59 | |
| VEGF mRNA | Young | 100.0 ± 24.9 | 128.0 ± 27.0 | 119.7 ± 24.9 | 0.17 | 0.15 |
| Old | 73.4 ± 18.4 | 196.0 ± 27.9 | 141.3 ± 23.7 | 0.00017 | 0.00019 | |
| Area of GFAP- immunopositive cells |
Young | 100.0 ± 14.8 | 141.4 ± 27.7 | 121.7 ± 20.6 | 0.022 | 0.29 |
| Old | 139.1 ± 23.1 | 216.8 ± 38.1 | 146.3 ± 28.4 | 0.017 | 0.043 | |
| Data are presented as percentages relative to young controls, whose values were taken as 100%. | ||||||
Administration of DHQ-WF for 8 weeks to aged LPS-treated animals resulted in a significant 25% (p < 0.05) decrease in vessel density and a non-significant decrease in vessel length.
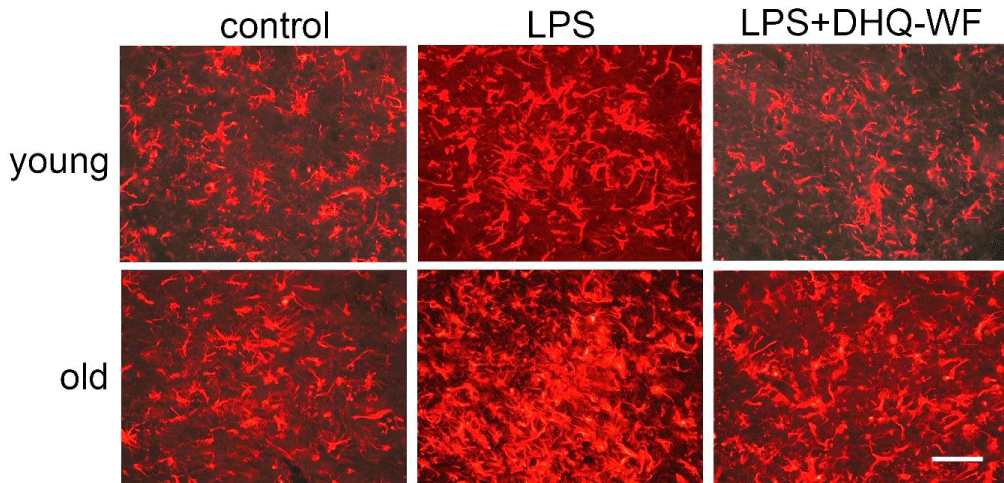
The levels of VEGF mRNA in the old control rats were significantly lower than in the young control rats (by 26.6%; p < 0.05). LPS treatment induced an increase in VEGF mRNA expression that was significant only in old rats in comparison to age-matched controls (by 122.6%; p < 0.001). Oral administration of DHQ-WF for 8 weeks to aged animals after LPS treatment significantly reduced VEGF mRNA expression by 54.7% (p < 0.01) compared with LPS-treated rats, although it did not reach the levels observed in control animals of similar age. No significant changes were observed in young animals under similar experimental conditions. The area occupied by GFAP-immunopositive bodies and processes in old animals was significantly higher than in young animals at 39.1% (p < 0.05) (Figure 2).
Figure 2: Photomicrographs of GFAP-immunopositive bodies and processes from young and aged rats labeled 8 weeks after administration of sterile saline (control), lipopolysaccharide (LPS), and lipopolysaccharide with simultaneous oral administration of a water-soluble form of dehydroquercetin (LPS+DHQ-WF). Line length = 80 µm.
Injection of LPS into the SN resulted in an excess of GFAP-immunopositive astroglial area in both age groups: by 41.4% (p < 0.05) in young and by 77.7% (p < 0.01) in old compared to age-matched controls. Oral administration of DHQ-WF reduced the cell area of astrocytes in both young (by 19.7%; p < 0.05) and old LPS-treated animals (by 70.5%; p < 0.01).
Analysis of microcirculatory morphology and GFAP expression in the SN of animals of different ages revealed significant age-related differences both in control and after a single injection of LPS, which is known to induce neuroinflammation [19,20]. Importantly, in old rats, even under normal physiological conditions, we observed signs of neuroinflammation in the SN: astrogliosis, as evidenced by a significant excess of areas occupied by GFAP-immunopositive cell bodies and processes, decreased density of microcirculatory vessels with their extended length, indicating an increase in their tortuosity compared to young animals. Such low-level age-related neuroinflammation has previously been referred to as “neuroinflammaging” [21]. This is consistent with observations of similar vascular changes in several regions of the aged brain in both humans and animals. [22- 28]. It was previously shown that the tortuosity index of cerebral collateral vessels in mice increased significantly with increasing relative resistance to blood flow [29]. The observed vascular changes, indicative of decreased mean blood flow velocity in the SN, may underlie the dysfunction of the Dopaminergic (DA) system, which is altered with normal aging [30]. Perfusion deficits have also been observed before the onset of clinical symptoms in other neurodegenerative disorders, which suggests that the deficits contribute to the pathogenesis of the disease [31].
The reduced expression of VEGF mRNA in the SN of the aged control rats compared to the relatively young control animals that were detected in our study may be indicative of a low level of angiogenesis in the SN of the aged rats. The results obtained are consistent with previous data suggesting that the mechanism responsible for impaired angiogenesis in aged animals is related to decreased VEGF expression [11,32].
It is possible that the observed changes in microvessels and astroglia during normal aging may contribute to the “sensitivity” of the age-related central nervous system to the action of additional pro-inflammatory factors. Indeed, we found that a single injection of LPS significantly increased blood microvessel density and VEGF mRNA expression in the SN of old, but not young, animals.
Administration of LPS into the SN-induced angiogenesis as evidenced by an increase in the number of microvessels. Our observation of LPS-induced angiogenesis is consistent with data that hypoxia or inflammation usually induces angiogenesis in the adult brain [33]. Increased nigral vascularization and VEGF levels after LPS administration may be a consequence of induced neuroinflammation because upregulation of VEGF has been reported in inflammatory processes [31]. Importantly, increased vascularization and upregulation of VEFG levels were observed in the SN of PD patients [14,34] and animal models of PD [10,35,36].
There is disagreement regarding the role of VEGF in the development of neuroinflammation and subsequent Parkinson’s disease. Several studies have shown that VEGF is a highly potent neuroprotective and neurorescue molecule for dopamine (DA) neurons [37]. However, recent studies have revealed that VEGF is also involved in neurodegeneration [31]. Thus, increased levels of VEGF in the Cerebrospinal Fluid (CSF) of PD patients have been suggested to be associated with Blood-Brain Barrier (BBB) dysfunction and neuronal degeneration [5]. It has also been suggested that VEGF has a “Janus face” because it has been shown that at different stages of stroke elevated VEGF levels cause destruction of the Blood-Brain Barrier (BBB) and disturbance of homeostasis or have neuroprotective effects [38].
Examination of GFAP-positive astroglia after nigral administration of LPS showed that it increased in young animals, but to a greater extent in old animals. Astrocytes are the most abundant cell type within the CNS, and they provide structural support, promote the formation of the BBB, and release beneficial factors that maintain brain cell development and homeostasis of the extracellular environment [39]. However, under pathological conditions, astrocytes secrete inflammatory factors, such as VEGF, chemokines, and cytokines; these factors directly or indirectly aggravate brain damage and BBB disruption [40]. Recently, it has been proposed that neuroinflammation induced two different types of reactive astrocytes, which are called toxic A1 astrocytes and neuroprotective A2 reactive astrocytes [41].
This suggests that the activation of astroglia in old animals in response to LPS administration is not only characterized by a greater intensity of astrogliosis but also probably by the formation of A1 cells that produce higher levels of VEGF, which in turn induces angiogenesis.
Daily oral administration of DHQ-WF for 8 weeks resulted in a significant reduction of LPS-induced astroglial cell activation, microcirculatory changes and reduced VEGF-A mRNA expression, which was more pronounced in old rats compared to young rats under the same experimental conditions. Such a pronounced ‘normalizing’ effect of DHQ-WF use allows us to consider it as a potent neuroprotective agent capable of reducing abnormalities in the bloodstream and glial microenvironment that lead to the manifestation of Parkinson’s disease.
Recommendations
The data obtained allow us to hypothesize that low-level neuroinflammation and aberrant regulation of VEGF, leading to a decrease in angiogenesis in old age, form an increased sensitivity of the SN to the action of additional inflammatory factors. This may lead to loss of blood-brain barrier integrity, microvascular leakage, increased neuroinflammation, and activation of a self-perpetuating vicious cycle of SN cell death. However, this hypothesis requires further experimental validation. Furthermore, prospective studies with appropriate power levels should be conducted based on this study to verify the conclusion that DHQ-WH is a promising neuro- and neuroprotective agent.
- Duggan M, Torkzaban B, Ahooyi TM, Khalili K, Gordon J. Age-related neurodegenerative diseases. J Cell Physiol. 2020 Apr;235(4):3131-3141. doi: 10.1002/jcp.29248. Epub 2019 Sep 25. PMID: 31556109; PMCID: PMC7029396.
- Collier TJ, Kanaan NM, Kordower JH. Aging and Parkinson's disease: Different sides of the same coin? Mov Disord. 2017 Jul;32(7):983-990. doi: 10.1002/mds.27037. Epub 2017 May 18. PMID: 28520211; PMCID: PMC5844262.
- Michel PP, Hirsch EC, Hunot S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron. 2016 May 18;90(4):675-91. doi: 10.1016/j.neuron.2016.03.038. PMID: 27196972.
- McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008 Mar 15;23(4):474-83. doi: 10.1002/mds.21751. PMID: 18044695.
- Janelidze S, Lindqvist D, Francardo V, Hall S, Zetterberg H, Blennow K, Adler CH, Beach TG, Serrano GE, van Westen D, Londos E, Cenci MA, Hansson O. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology. 2015 Nov 24;85(21):1834-42. doi: 10.1212/WNL.0000000000002151. Epub 2015 Oct 28. PMID: 26511451; PMCID: PMC4662706.
- Paul G, Elabi OF. Microvascular Changes in Parkinson's Disease- Focus on the Neurovascular Unit. Front Aging Neurosci. 2022 Mar 10; 14:853372. doi: 10.3389/fnagi.2022.853372. PMID: 35360216; PMCID: PMC8960855.
- Padel T, Özen I, Boix J, Barbariga M, Gaceb A, Roth M, Paul G. Platelet-derived growth factor-BB has neurorestorative effects and modulates the pericyte response in a partial 6-hydroxydopamine lesion mouse model of Parkinson's disease. Neurobiol Dis. 2016 Oct; 94:95-105. doi: 10.1016/j.nbd.2016.06.002. Epub 2016 Jun 7. PMID: 27288154.
- Elabi OF, Cunha JPMCM, Gaceb A, Fex M, Paul G. High-fat diet-induced diabetes leads to vascular alterations, pericyte reduction, and perivascular depletion of microglia in a 6-OHDA toxin model of Parkinson disease. J Neuroinflammation. 2021 Aug 10;18(1):175. doi: 10.1186/s12974-021-02218-8. PMID: 34376193; PMCID: PMC8353816.
- Faucheux BA, Bonnet AM, Agid Y, Hirsch EC. Blood vessels change in the mesencephalon of patients with Parkinson's disease. Lancet. 1999 Mar 20;353(9157):981-2. doi: 10.1016/S0140-6736(99)00641-8. PMID: 10459912.
- Barcia C, Bautista V, Sánchez-Bahillo A, Fernández-Villalba E, Faucheux B, Poza y Poza M, Fernandez Barreiro A, Hirsch EC, Herrero MT. Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. J Neural Transm (Vienna). 2005 Sep;112(9):1237-48. doi: 10.1007/s00702-004-0256-2. Epub 2005 Jan 24. PMID: 15666038.
- Wang H, Keiser JA, Olszewski B, Rosebury W, Robertson A, Kovesdi I, Gordon D. Delayed angiogenesis in aging rats and therapeutic effect of adenoviral gene transfer of VEGF. Int J Mol Med. 2004 Apr;13(4):581-7. PMID: 15010860.
- Lange C, Storkebaum E, de Almodóvar CR, Dewerchin M, Carmeliet P. Vascular endothelial growth factor: a neurovascular target in neurological diseases. Nat Rev Neurol. 2016 Aug;12(8):439-54. doi: 10.1038/nrneurol.2016.88. Epub 2016 Jul 1. PMID: 27364743.
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359-71. doi: 10.1038/nrm1911. PMID: 16633338.
- Wada K, Arai H, Takanashi M, Fukae J, Oizumi H, Yasuda T, Mizuno Y, Mochizuki H. Expression levels of vascular endothelial growth factor and its receptors in Parkinson's disease. Neuroreport. 2006 May 15;17(7):705-9. doi: 10.1097/01.wnr.0000215769.71657.65. PMID: 16641673.
- Yang R, Yang X, Zhang F. New Perspectives of Taxifolin in Neurodegenerative Diseases. Curr Neuropharmacol. 2023;21(10):2097-2109. doi: 10.2174/1570159X21666230203101107. PMID: 36740800; PMCID: PMC10556370.
- Zhang X, Lian X, Li H, Zhao W, Li X, Zhou F, Zhou Y, Cui T, Wang Y, Liu C. Taxifolin attenuates inflammation via suppressing MAPK signal pathway in vitro and in silico analysis. Chin Herb Med. 2022 Sep 1;14(4):554-562. doi: 10.1016/j.chmed.2021.03.002. PMID: 36405054; PMCID: PMC9669345.
- Varlamova EG, Uspalenko NI, Khmil NV, Shigaeva MI, Stepanov MR, Ananyan MA, Timchenko MA, Molchanov MV, Mironova GD, Turovsky EA. A Comparative Analysis of Neuroprotective Properties of Taxifolin and Its Water-Soluble Form in Ischemia of Cerebral Cortical Cells of the Mouse. Int J Mol Sci. 2023 Jul 14;24(14):11436. doi: 10.3390/ijms241411436. PMID: 37511195; PMCID: PMC10380368.
- Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6(11):e27385. doi: 10.1371/journal.pone.0027385. Epub 2011 Nov 16. PMID: 22110636; PMCID: PMC3217985.
- Lopes PC. LPS and neuroinflammation: a matter of timing. Inflammopharmacology. 2016 Oct;24(5):291-293. doi: 10.1007/s10787-016-0283-2. Epub 2016 Sep 19. PMID: 27645902.
- Li B, Wang M, Chen S, Li M, Zeng J, Wu S, Tu Y, Li Y, Zhang R, Huang F, Tong X. Baicalin Mitigates the Neuroinflammation through the TLR4/MyD88/NF-κB and MAPK Pathways in LPS-Stimulated BV-2 Microglia. Biomed Res Int. 2022 Nov 9; 2022:3263446. doi: 10.1155/2022/3263446. PMID: 36408278; PMCID: PMC9668451.
- Pizza V, Agresta A, D'Acunto CW, Festa M, Capasso A. Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS Neurol Disord Drug Targets. 2011 Aug;10(5):621-34. doi: 10.2174/187152711796235014. PMID: 21631404.
- Desjardins M, Berti R, Lefebvre J, Dubeau S, Lesage F. Aging-related differences in cerebral capillary blood flow in anesthetized rats. Neurobiol Aging. 2014 Aug;35(8):1947-55. doi: 10.1016/j.neurobiolaging.2014.01.136. Epub 2014 Jan 31. PMID: 24612672.
- Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007 Jun 15;257(1-2):62-6. doi: 10.1016/j.jns.2007.01.015. Epub 2007 Feb 23. PMID: 17320909; PMCID: PMC1989116.
- Casey MA, Feldman ML. Aging in the rat medial nucleus of the trapezoid body. III. Alterations in capillaries. Neurobiol Aging. 1985 Spring;6(1):39-46. doi: 10.1016/0197-4580(85)90070-3. PMID: 4000384.
- Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging. 1981 Winter;2(4):283-91. doi: 10.1016/0197-4580(81)90037-3. PMID: 7335147.
- Bell MA, Ball MJ. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol. 1981;53(4):299-318. doi: 10.1007/BF00690372. PMID: 7223373.
- Lowerison MR, Sekaran NVC, Zhang W, Dong Z, Chen X, Llano DA, Song P. Aging-related cerebral microvascular changes visualized using ultrasound localization microscopy in the living mouse. Sci Rep. 2022 Jan 12;12(1):619. doi: 10.1038/s41598-021-04712-8. PMID: 35022482; PMCID: PMC8755738.
- Murugesan N, Demarest TG, Madri JA, Pachter JS. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging. 2012 May; 33(5):1004.e1-16. doi: 10.1016/j.neurobiolaging.2011.09.022. Epub 2011 Oct 21. PMID: 22019053; PMCID: PMC3266473.
- Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011 Aug;31(8):1748-56. doi: 10.1161/ATVBAHA.111.227314. Epub 2011 May 26. PMID: 21617137; PMCID: PMC3141082.
- Schallert T. Aging-dependent emergence of sensorimotor dysfunction in rats recovered from dopamine depletion sustained early in life. Ann N Y Acad Sci. 1988; 515:108-20. doi: 10.1111/j.1749-6632.1988.tb32972.x. PMID: 3364880.
- Storkebaum E, Carmeliet P. VEGF: a critical player in neurodegeneration. J Clin Invest. 2004 Jan;113(1):14-8. doi: 10.1172/JCI20682. PMID: 14702101; PMCID: PMC300888.
- Villar-Cheda B, Sousa-Ribeiro D, Rodriguez-Pallares J, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson's disease. J Cereb Blood Flow Metab. 2009 Feb;29(2):230-4. doi: 10.1038/jcbfm.2008.127. Epub 2008 Oct 29. PMID: 18957989.
- Tahergorabi Z, Khazaei M. A review on angiogenesis and its assays. Iran J Basic Med Sci. 2012 Nov;15(6):1110-26. PMID: 23653839; PMCID: PMC3646220.
- Desai Bradaric B, Patel A, Schneider JA, Carvey PM, Hendey B. Evidence for angiogenesis in Parkinson's disease, incidental Lewy body disease, and progressive supranuclear palsy. J Neural Transm (Vienna). 2012 Jan;119(1):59-71. doi: 10.1007/s00702-011-0684-8. Epub 2011 Jul 12. PMID: 21748523; PMCID: PMC3352316.
- Elabi O, Gaceb A, Carlsson R, Padel T, Soylu-Kucharz R, Cortijo I, Li W, Li JY, Paul G. Human α-synuclein overexpression in a mouse model of Parkinson's disease leads to vascular pathology, blood brain barrier leakage and pericyte activation. Sci Rep. 2021 Jan 13;11(1):1120. doi: 10.1038/s41598-020-80889-8. PMID: 33441868; PMCID: PMC7806665.
- Tahergorabi Z, Khazaei M. A review on angiogenesis and its assays. Iran J Basic Med Sci. 2012 Nov;15(6):1110-26. PMID: 23653839; PMCID: PMC3646220.
- Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, Matsui T, Miyoshi Y, Hamada H, Date I. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson's disease. Eur J Neurosci. 2004 Mar;19(6):1494-504. doi: 10.1111/j.1460-9568.2004.03254.x. PMID: 15066146.
- Geiseler SJ, Morland C. The Janus Face of VEGF in Stroke. Int J Mol Sci. 2018 May 4;19(5):1362. doi: 10.3390/ijms19051362. PMID: 29734653; PMCID: PMC5983623.
- Ransom BR, Ransom CB. Astrocytes: multitalented stars of the central nervous system. Methods Mol Biol. 2012;814:3-7. doi: 10.1007/978-1-61779-452-0_1. PMID: 22144296.
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012 Jul;122(7):2454-68. doi: 10.1172/JCI60842. Epub 2012 Jun 1. PMID: 22653056; PMCID: PMC3386814.
- Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017 Jun 20;46(6):957-967. doi: 10.1016/j.immuni.2017.06.006. PMID: 28636962.