More Information
Submitted: February 13, 2024 | Approved: February 21, 2024 | Published: February 22, 2024
How to cite this article: Freeze R, Scarneo S. Chemotherapy-induced Peripheral Neuropathy: A Mini-review of Current & Developmental Treatments. J Neurosci Neurol Disord. 2024; 8: 020-023.
DOI: 10.29328/journal.jnnd.1001093
Copyright License: © 2024 Freeze R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Neuropathy; Chemotherapeutics; Drug development
Chemotherapy-induced Peripheral Neuropathy: A Mini-review of Current & Developmental Treatments
Robert Freeze and Scott Scarneo*
and Scott Scarneo*
EydisBio Inc., Durham NC 27701, USA
*Address for Correspondence: Scott Scarneo, EydisBio Inc., Durham NC 27701, USA, Email: [email protected]
Chemotherapy-Induced Peripheral Neuropathy (CIPN) is a major limiting side effect of many common chemotherapeutics often leading patients to terminate their chemotherapy treatment regimen early. The development of CIPN differs by chemotherapeutic class, with platinum- and taxane-based treatments demonstrating the highest incidence rates. Despite its relatively high prevalence, there are currently no FDA-approved treatments for CIPN, and clinicians must rely on the off-label use of several analgesics and various non-pharmacological approaches to treat CIPN symptoms in patients. Novel insights on the development of CIPN have identified new drug targets leading to several Phase II clinical trials to be initiated. Here, we describe recent advances in drug development for CIPN.
Chemotherapy-Induced Peripheral Neuropathy (CIPN) [1] is a common and debilitating side effect of certain neurotoxic cancer drugs such as platinum-based compounds, taxanes, vinca alkaloids, and bortezomib [2]. CIPN can cause pain, numbness, tingling, and loss of function in the hands and feet, affecting the quality of life and survival rates of cancer patients [3]. In 2023, there were approximately 2.0 million new cases of cancer reported within the US, and it has been reported that approximately 58% of patients require some form of chemotherapy [4,5]. Of these, neurotoxic chemotherapies such as platinum- and taxane-based drugs are commonly used, and it has been estimated that 50% - 70% of cancer patients receive platinum-based therapy as part of their treatment regimen [6]. In a 2014 study, researchers examined a cohort of 4,179 cancer patients across 30 clinical studies who were treated with various neurotoxic chemotherapies, their findings of which revealed a combined CIPN prevalence of 48% among these patients [7]. Prevalence varied based on the timeframe post-treatment where approximately 68% of patients experienced CIPN after 1 month, 60% after 3 months, and 30% after 6 months following treatment [7]. However, CIPN prevalence differs based on chemotherapy type, with oxaliplatin exhibiting the highest CIPN rate at around 71% and paclitaxel at 63% measured at 6 months post-treatment [8]. Another review describes agent-dependent CIPN prevalence of 70% - 100% for platinum-based drugs, 11% - 87% for taxanes, and 20% - 60% for thalidomide and its analogues. The rate of CIPN in bortezomib-treated patients is approximately 27% [9,10].
There is no definitive or approved prevention or treatment for CIPN, but some strategies that have been studied include dose modification, pharmacological agents, and non-pharmacological interventions [11-13]. The only pharmacological agent recommended for treating CIPN pain to date is the Serotonin and Norepinephrine Reuptake Inhibitor (SNRI) antidepressant, duloxetine, however even its benefit is limited [11,14,15]. For example, one study comparing duloxetine to placebo for both the prevention and treatment of CIPN showed duloxetine’s effect to be statistically similar to placebo. An alternative study suggested that duloxetine’s highest approved dose of 60 mg was actually inferior to pregabalin’s approved mid-dose of 150 mg in taxane-induced peripheral neuropathy [16-20]. Other drugs that may provide CIPN analgesia include opioids, steroids, topical anesthetics, and anticonvulsants [15]. Non-pharmacological interventions that may improve CIPN symptoms include physical therapy, occupational therapy, acupuncture, electrical nerve stimulation, and biofeedback [14]. However, the evidence for these modalities is limited, and more clinical data is needed to establish their efficacy and safety.
Novel, non-opioid therapeutic targets for CIPN
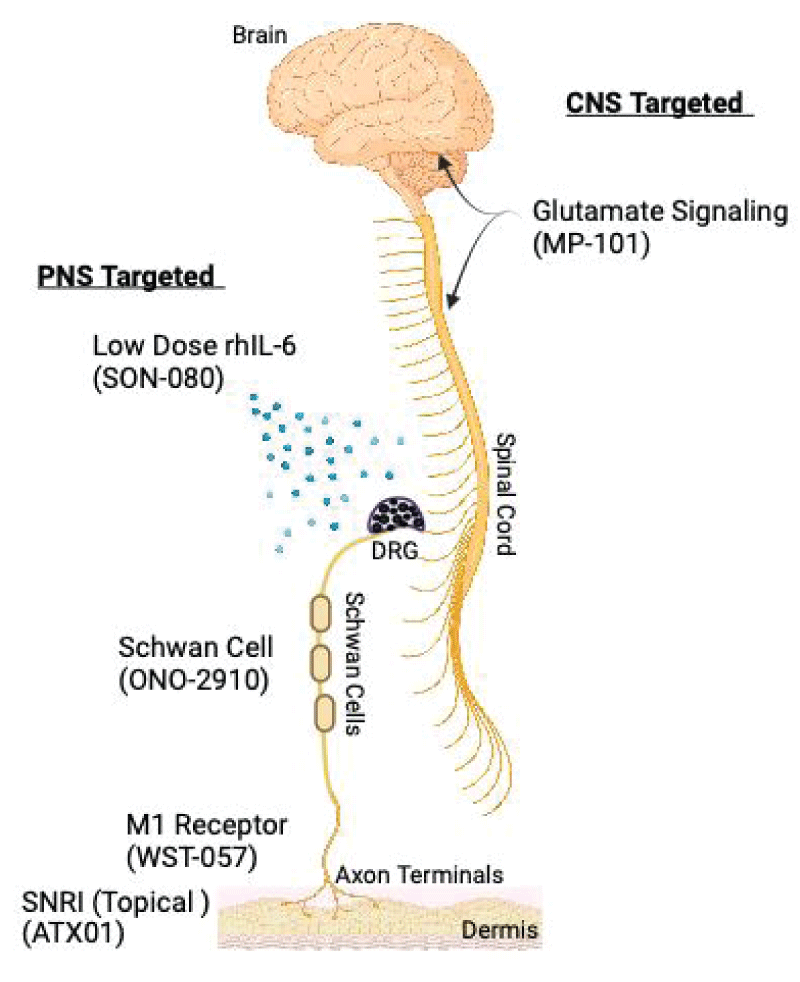
Despite the advances in understanding the pathophysiology of CIPN, there is still a lack of effective and safe therapies that are specifically approved for this condition. Therefore, there is a need to explore new mechanisms of action and developmental products that target both the prevention and treatment of CIPN. Emerging approaches span a variety of mechanisms of action, the most developed of which include, IL-6 antagonists, Schwann cell differentiation enhancers, glutamate signaling modulators, Histone Deacetylase 6 (HDAC6) inhibitors, muscarinic M1 receptor antagonists, apoptosis stimulants, and SNRIs (Figure 1). Among these are both small molecules formulated as either orals or topicals, as well as injectable biologics. Here, and summarized in Table 1,
Figure 1: Peripheral and central nervous system targets of Phase II CIPN treatments. Peripheral Nervous System (PNS) targeted therapies include, SON-080 a low-dose recombinant human IL-6 (rhIL-6), ONO-2910 which enhances Schwan cell myelination, WST-057 which increases axon terminal density and ATX01 a topical SNRI. Central Nervous System (CNS) therapy includes MP-101 an NMDA receptor antagonist. DRG = Dorsal Root Ganglia.
| Table 1: Select Therapeutic Approaches Currently in Phase 1 & Phase 2 Clinical Development for CIPN | |||||||
| Drug | MoA | Company | Phase | Molecule Type | Route of Administration | Intervention Type | Patient Population |
| ONO-2910 | Enhancement of Schwann cell differentiation | Ono Pharmaceutical | Phase 2 | Small | Oral | Prevention | Breast cancer patients |
| MP-101 | Glutamate signaling modulator (acetylcholine receptor agonist; NMDA receptor antagonist) | Novaremed | Phase 2 | Small | Oral | Prevention | CIPN patients |
| ATX01 | Serotonin & norepinephrine reuptake inhibitor | Algo Therapeutix |
Phase 2 | Small | Topical | Treatment | CIPN patients |
| WST-057 | Muscarinic M1 receptor antagonist | WinSanTor | Phase 2 | Small | Topical | Treatment | CIPN patients |
| SON-080 | IL-6 antagonist | Sonnet BioTherapeutics | Phase 1/2 | Biologic | Injectable | Treatment | CIPN patients |
| BXQ-350 | Apoptosis stimulant | Bexion Pharmaceuticals | Phase 1 | Biologic | Injectable | Treatment | CIPN patients |
| Ricolinostat | Histone deacetylase 6 (HDAC6) inhibitor | Regenacy Pharmaceuticals | Phase 1 | Small | Oral | Treatment | CIPN patients |
we describe some select recent advances in CIPN-targeted therapeutics and their current developmental status.
Therapeutic approaches currently in phase 2 clinical development
Schwann Cell Differentiation Enhancement. Ono Pharmaceuticals (Japan) is developing small molecule ONO-2910 outside of the US as an orally bioavailable treatment for CIPN pain [21]. ONO-2910 accelerates the repair process of nerve fibers by enhancing Schwann cell differentiation into myelin-forming cells and suppressing the de-differentiation into injury-responsive cells [22]. By doing so, ONO-2910 may prevent or reduce the onset and severity of CIPN symptoms. ONO-2910 is currently recruiting for a Phase 2 clinical trial to investigate its use as a prevention of CIPN in breast cancer patients receiving weekly paclitaxel [23].
Glutamate Signaling Modulation. MP-101 is a small molecule, orally administered glutamate signaling modulator that acts as an NMDA receptor antagonist and contains racemic dimiracetam and its R-enantiomer as active ingredients. Excessive glutamate signaling can lead to neuronal damage and pain sensitization, and MP-101 works by blocking the release of glutamate from nerve terminals and reducing the activity of NMDA receptors, which are key mediators of glutamate signaling [24]. MP-101 is being developed by Novaremed (Switzerland) for the prevention of CIPN pain [25].
Serotonin and Norepinephrine Reuptake Inhibition. As an SNRI, ATX01 aims to improve upon fellow SNRI duloxetine’s effectiveness, which is currently used off-label to treat CIPN, but as an approved drug. AlgoTherapeutix (France) is developing ATX01 as a topical formulation of amitriptyline to target nerve fibers in the skin and avoid potential systemic toxicities and has received Fast-Track designation from the U.S. Food and Drug Administration (FDA) [26]. AlgoTherapeutic is currently enrolling for a global Phase II study of ATX01 in CIPN patients [27].
Muscarinic M1 Receptors Antagonization. WST-057 is a muscarinic M1 receptor antagonist being developed as a treatment for CIPN. WST-057 is being developed by WinSanTor (US) as a topical formulation of pirenzepine, which was originally approved for use in Europe and Asia to treat gastric ulcers but has yet to be approved by the FDA for CIPN pain. WST-057 acts to block the action of acetylcholine to prevent or reduce nerve damage and pain caused by CIPN and may have neuroprotective effects by enhancing nerve growth factor signaling and increasing nerve fiber density [28]. WST-057 is currently recruiting for a Phase 2 clinical trial in patients with CIPN [29].
Low-Dose IL-6 Mimetic. Sonnet Biotherapeutics (US) is currently developing an injectable, low-dose IL-6 antagonist (SON-080) for the treatment of CIPN pain [30]. At high expression levels, IL-6 acts as a proinflammatory cytokine. However, as a recombinant human IL-6 mimetic, SON-080 works by mimicking the physiological effects of low levels of native IL-6 which can reduce inflammation, promote nerve regeneration, and improve glucose homeostasis. Sonnet is currently recruiting for a Phase 1/2 trial for SON-080 in CIPN patients [31].
Therapeutic approaches currently in phase 1 clinical development
Histone Deacetylase 6 Inhibition. Regenacy Pharmaceuticals (US) is currently developing ricolinostat, an aHDAC6 inhibitor, as an orally bioavailable treatment for CIPN pain. Ricolinostat works by inhibiting the HDAC6 enzyme, which removes acetyl groups from proteins, and HDAC6 specifically is a key regulator of various mitochondrial mechanisms, dysfunction of which can lead to peripheral neuropathy [32]. Also, by selectively inhibiting HDAC6 as opposed to pan-HDAC inhibition by drugs such as vorinostat and panobinostat, ricolinostat may resultingly confer a lower risk of side effects [33]. It may also have neuroprotective effects. Regenacy Pharmaceuticals is currently recruiting for a Phase 1 CIPN trial for the use of ricolinostat in breast cancer patients previously treated with docetaxel or paclitaxel [34].
Apoptosis Stimulation. BXQ-350 is an apoptosis stimulant being developed by Bexion Pharmaceuticals (US). BXQ-350 may have neuroprotective effects by reducing the levels of Sphingosine-1-Phosphate (S1P), an anti-apoptotic sphingolipid that promotes cell survival, proliferation, and migration, as well as immune suppression and inflammation [35]. S1P has also been implicated in the development and maintenance of neuropathic pain, as it selectively activates the S1P receptor subtype 1 in astrocytes to modulate neuro-immune cell interactions [36,37]. Neuroinflammation, triggered by elevated proinflammatory cytokines within the spinal cord, most notably TNFα and IL-1β, plays a pivotal role in the initiation and persistence of neuropathic pain [38]. Specifically, IL-1β enhances neuronal excitability, thereby contributing to central sensitization during neuropathic pain, partly through its inhibitory effect on IL-10 release [38].
Here, we describe the current efforts of several clinical-stage CIPN-targeted programs. Despite several drugs in Phase 2 clinical trials, high historical failure rates of drugs to advance from Phase 2 to approval means it is likely that many, if not most, of these drugs will never make it to market and into clinical use. In fact, the likelihood of a neuro-targeted drug in Phase 2 clinical development reaching approval is approximately 24%, which is less than the 27% average for non-oncology drugs in general [39]. Thus, there is a large need for other novel therapeutic targets to be identified which can expand our efforts to better prevent and treat CIPN as well as expand our drug armamentarium.
Conflict of interest
Robert Freeze and Scott Scarneo are both shareholders in EydisBio, Inc., which is currently developing novel treatments for CIPN.
- Ibrahim EY, Ehrlich BE. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit Rev Oncol Hematol. 2020 Jan;145:102831. doi: 10.1016/j.critrevonc.2019.102831. Epub 2019 Nov 13. PMID: 31783290; PMCID: PMC6982645.
- Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. 2017 Jun;81(6):772-781. doi: 10.1002/ana.24951. Epub 2017 Jun 5. PMID: 28486769; PMCID: PMC5656281.
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006 Feb;33(1):15-49. doi: 10.1053/j.seminoncol.2005.12.010. PMID: 16473643.
- Wilson BE, Jacob S, Yap ML, Ferlay J, Bray F, Barton MB. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: a population-based study. Lancet Oncol. 2019 Jun;20(6):769-780. doi: 10.1016/S1470-2045(19)30163-9. Epub 2019 May 8. Erratum in: Lancet Oncol. 2019 Jul;20(7):e346. PMID: 31078462.
- Research. A.A.f.C. Cancer in 2023.
- Armstrong-Gordon E, Gnjidic D, McLachlan AJ, Hosseini B, Grant A, Beale PJ, Wheate NJ. Patterns of platinum drug use in an acute care setting: a retrospective study. J Cancer Res Clin Oncol. 2018 Aug;144(8):1561-1568. doi: 10.1007/s00432-018-2669-6. Epub 2018 May 22. PMID: 29789926.
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014 Dec;155(12):2461-2470. doi: 10.1016/j.pain.2014.09.020. Epub 2014 Sep 23. PMID: 25261162.
- Molassiotis A, Cheng HL, Lopez V, Au JSK, Chan A, Bandla A, Leung KT, Li YC, Wong KH, Suen LKP, Chan CW, Yorke J, Farrell C, Sundar R. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019 Feb 8;19(1):132. doi: 10.1186/s12885-019-5302-4. PMID: 30736741; PMCID: PMC6368751.
- Giraudet F, Selvy M, Kerckhove N, Pereira B, Barreau F, Nguyen D, Busserolles J, Cabrespine A, Chaleteix C, Soubrier M, Bay JO, Lemal R, Balayssac D. Relation between auditory difficulties and bortezomib-induced peripheral neuropathy in multiple myeloma: a single-center cross-sectional study. Eur Arch Otorhinolaryngol. 2022 Apr;279(4):2197-2201. doi: 10.1007/s00405-021-07234-1. Epub 2022 Jan 31. PMID: 35098333.
- Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci. 2019 Mar 22;20(6):1451. doi: 10.3390/ijms20061451. PMID: 30909387; PMCID: PMC6471666.
- Mezzanotte JN, Grimm M, Shinde NV, Nolan T, Worthen-Chaudhari L, Williams NO, Lustberg MB. Updates in the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Curr Treat Options Oncol. 2022 Jan;23(1):29-42. doi: 10.1007/s11864-021-00926-0. Epub 2022 Feb 15. PMID: 35167004; PMCID: PMC9642075.
- Hu S, Huang KM, Adams EJ, Loprinzi CL, Lustberg MB. Recent Developments of Novel Pharmacologic Therapeutics for Prevention of Chemotherapy-Induced Peripheral Neuropathy. Clin Cancer Res. 2019 Nov 1;25(21):6295-6301. doi: 10.1158/1078-0432.CCR-18-2152. Epub 2019 May 23. PMID: 31123053; PMCID: PMC6825524.
- Sharma MR, Mehrotra S, Gray E, Wu K, Barry WT, Hudis C, Winer EP, Lyss AP, Toppmeyer DL, Moreno-Aspitia A, Lad TE, Velasco M, Overmoyer B, Rugo HS, Ratain MJ, Gobburu JV. Personalized Management of Chemotherapy-Induced Peripheral Neuropathy Based on a Patient Reported Outcome: CALGB 40502 (Alliance). J Clin Pharmacol. 2020 Apr;60(4):444-452. doi: 10.1002/jcph.1559. Epub 2019 Dec 4. PMID: 31802506; PMCID: PMC7064382.
- Brown TJ, Sedhom R, Gupta A. Chemotherapy-Induced Peripheral Neuropathy. JAMA Oncol. 2019 May 1;5(5):750. doi: 10.1001/jamaoncol.2018.6771. PMID: 30816956.
- Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, Kelley MR, Lavino A, Lustberg MB, Paice JA, Schneider BP, Lavoie Smith EM, Smith ML, Smith TJ, Wagner-Johnston N, Hershman DL. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J Clin Oncol. 2020 Oct 1;38(28):3325-3348. doi: 10.1200/JCO.20.01399. Epub 2020 Jul 14. PMID: 32663120.
- Salehifar E, Janbabaei G, Hendouei N, Alipour A, Tabrizi N, Avan R. Comparison of the Efficacy and Safety of Pregabalin and Duloxetine in Taxane-Induced Sensory Neuropathy: A Randomized Controlled Trial. Clin Drug Investig. 2020 Mar;40(3):249-257. doi: 10.1007/s40261-019-00882-6. PMID: 31925721.
- Chow R, Novosel M, So OW, Bellampalli S, Xiang J, Boldt G, Winquist E, Lock M, Lustberg M, Prsic E. Duloxetine for prevention and treatment of chemotherapy-induced peripheral neuropathy (CIPN): systematic review and meta-analysis. BMJ Support Palliat Care. 2023 Mar;13(1):27-34. doi: 10.1136/spcare-2022-003815. Epub 2022 Sep 8. PMID: 36194493.
- Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL; Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013 Apr 3;309(13):1359-67. doi: 10.1001/jama.2013.2813. PMID: 23549581; PMCID: PMC3912515.
- Cymbalta FDA Label. (fda.gov).
- Lyrica FDA Label (fda.gov).
- Dedicated to the Fight Against Disease and Pain. https://us.ono-pharma.com
- Boerboom A, Dion V, Chariot A, Franzen R. Molecular Mechanisms Involved in Schwann Cell Plasticity. Front Mol Neurosci. 2017 Feb 17;10:38. doi: 10.3389/fnmol.2017.00038. PMID: 28261057; PMCID: PMC5314106.
- ONO-2910-03: An Early Phase II Study to Investigate the Chemotherapy-Induced Peripheral Neuropathy (CIPN) Onset-Suppressing Effect of ONO-2910 in Patients With Breast Cancer Receiving Paclitaxel. . Japan Registry of Clinical Trials JRCT ID: jRCT2031230173.
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002 May;5(5):405-14. doi: 10.1038/nn835. PMID: 11953750.
- Novaremed Non Opioid Treatment for Pain. https://www.novaremed.com
- AlgoTx. AlgoTx Announces Initiation of Global Phase 2 Clinical Trial of ATX01 for the Relief of Pain in Chemotherapy-induced Peripheral Neuropathy in Adults – AlgoTx. 2022.
- ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. NCT05593614
- Naznin F, Waise TMZ, Fernyhough P. Antagonism of the Muscarinic Acetylcholine Type 1 Receptor Enhances Mitochondrial Membrane Potential and Expression of Respiratory Chain Components via AMPK in Human Neuroblastoma SH-SY5Y Cells and Primary Neurons. Mol Neurobiol. 2022 Nov;59(11):6754-6770. doi: 10.1007/s12035-022-03003-1. Epub 2022 Aug 25. PMID: 36002781; PMCID: PMC9525428.
- A Study of Topical Pirenzepine or Placebo in Oncology Patients With Chemotherapy Induced Peripheral Neuropathy. NCT05488873.
- Sonnet Biotherapeutics. www.sonnetbio.com/pipeline.
- SON-080 in Patients With Persistent Chemotherapy-induced Peripheral Neuropathy (CIPN). NCT05435742
- English K, Barton MC. HDAC6: A Key Link Between Mitochondria and Development of Peripheral Neuropathy. Front Mol Neurosci. 2021 Aug 31;14:684714. doi: 10.3389/fnmol.2021.684714. PMID: 34531721; PMCID: PMC8438325.
- Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals (Basel). 2010 Aug 26;3(9):2751-2767. doi: 10.3390/ph3092751. PMID: 27713375; PMCID: PMC4034096.
- A Study to Investigate the Safety and Efficacy of Ricolinostat. NCT05229042.
- Weigert A, Olesch C, Brüne B. Sphingosine-1-Phosphate and Macrophage Biology-How the Sphinx Tames the Big Eater. Front Immunol. 2019 Jul 19;10:1706. doi: 10.3389/fimmu.2019.01706. PMID: 31379883; PMCID: PMC6658986.
- Chen Z, Doyle TM, Luongo L, Largent-Milnes TM, Giancotti LA, Kolar G, Squillace S, Boccella S, Walker JK, Pendleton A, Spiegel S, Neumann WL, Vanderah TW, Salvemini D. Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10557-10562. doi: 10.1073/pnas.1820466116. Epub 2019 May 8. PMID: 31068460; PMCID: PMC6534990.
- Salvemini D, Doyle TM. Targeting neuroinflammation in neuropathic pain and opioid use. J Exp Med. 2023 Feb 6;220(2):e20221244. doi: 10.1084/jem.20221244. Epub 2022 Dec 23. PMID: 36562735; PMCID: PMC9793426.
- Janes K, Little JW, Li C, Bryant L, Chen C, Chen Z, Kamocki K, Doyle T, Snider A, Esposito E, Cuzzocrea S, Bieberich E, Obeid L, Petrache I, Nicol G, Neumann WL, Salvemini D. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J Biol Chem. 2014 Jul 25;289(30):21082-97. doi: 10.1074/jbc.M114.569574. PMID: 24876379; PMCID: PMC4110312.
- Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019 Apr 1;20(2):273-286. doi: 10.1093/biostatistics/kxx069. Erratum in: Biostatistics. 2019 Apr 1;20(2):366. PMID: 29394327; PMCID: PMC6409418.