More Information
Submitted: March 07, 2024 | Approved: April 03, 2024 | Published: April 04, 2024
How to cite this article: Kharat S, Mali S, Korade G, Gaykar R. Navigating Neurodegenerative Disorders: A Comprehensive Review of Current and Emerging Therapies for Neurodegenerative Disorders. J Neurosci Neurol Disord. 2024; 8: 033-046.
DOI: 10.29328/journal.jnnd.1001095
Copyright License: © 2024 Kharat S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Neurodegenerative disorders; Therapeutic interventions; Gene therapy; Stem cell therapy; Emerging treatments’ RNAi; Neurotropic Factors
Navigating Neurodegenerative Disorders: A Comprehensive Review of Current and Emerging Therapies for Neurodegenerative Disorders
Shashikant Kharat*, Sanjana Mali*, Gayatri Korade and Rakhi Gaykar
Dr. DY. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune-18, India
*Address for Correspondence: Shashikant Kharat, Dr. DY. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune-18, India,
Email: [email protected]
Sanjana Mali, Dr. DY. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune-18, India
Neurodegenerative disorders (NDDs) pose a significant global health challenge, impacting millions with a gradual decline in neurons and cognitive abilities. Presently, available NDD therapies focus on symptom management rather than altering the disease trajectory. This underscores the critical necessity for groundbreaking treatments capable of addressing the root causes of neurodegeneration, offering both neuroprotection and neuro-restoration. This in-depth review delves into the forefront of emerging NDD therapies, encompassing gene therapy, stem cell therapy, immunotherapy, and neurotrophic factors. It sheds light on their potential advantages, hurdles, and recent advancements gleaned from both preclinical and clinical studies. Additionally, the document outlines existing NDD treatments, spanning pharmacological and non-pharmacological interventions, along with their inherent limitations. The overarching conclusion emphasizes the immense potential of emerging therapies in NDD treatment, yet underscores the imperative for continued research and optimization to ensure their safety, efficacy, and specificity.
Neurodegenerative Diseases (NDDs) represent a diverse spectrum of neurological disorders with a profound global impact, affecting millions worldwide. Characterized by the gradual decline of neurons in either the Central Nervous System (CNS) or Peripheral Nervous System (PNS), neurodegeneration stands as the central pathological feature in these conditions [1,2]. Oxidative and nitrosative stress, which is characterized by an excess of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) and a decline in cellular antioxidant defence systems, is the main cause of neuronal cell damage that results in NDDs [3]. NDDs, often referred to as NDs, are distinguished from static neuronal loss brought on by metabolic or toxic causes by the gradual loss of particular neuronal groupings. Primary clinical symptoms, key genetic abnormalities, or anatomical locations of neurodegeneration can all be used to categorize these diseases [4]. Among these, prion disease, motor neuron disease, Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, spinal muscular atrophy, and spinocerebellar ataxia are prominent. In 2019, these 18 major neurological disorders collectively contributed to 10.06 million deaths and 349.22 million Disability-Adjusted Life Years (DALYs) globally, ranking second only to cardiovascular diseases (excluding stroke). The burden of these disorders varied across regions, with significant reductions observed in South America, Asia, the Malay Archipelago, and much of Central Africa [5]. Traditional treatments, such as cholinesterase inhibitors for Alzheimer’s Disease (AD) or Levo-dopa for Parkinson’s Disease (PD), offer symptomatic relief but do not effectively address disease progression. Advances in understanding the neuro-molecular mechanisms of neurodegenerative diseases have led to the development of novel drugs aiming to mitigate the pathological aggregation of proteins [6]. Therapeutic strategies for neurodegenerative disorders focus on slowing degeneration and enhancing patients’ quality of life due to the irreversible nature of nervous tissue degeneration. Pharmacotherapy targets degenerative processes, altering neuron and glial cell metabolism, and compensating for lost functions. Non-pharmacological interventions, including comprehensive rehabilitation, aerobic activity, cognitive training, and occupational therapy, aim to improve adaptation to the environment and enhance daily functioning. Brain stimulation methods may also be considered [7]. Despite these efforts, effective therapeutic agents remain elusive due to the obscure causes of neuronal death and the challenges in early diagnosis. Promisingly, stem cells and neurotrophic factors emerge as potential therapeutic agents with neural differentiation and neuroprotective effects for neurodegenerative diseases [8,9]. While some strategies are in experimental stages, they hold the promise of transformative advancements in neurodegenerative disorder treatments [8,9].
Amyotrophic lateral sclerosis
Amyotrophic Lateral Sclerosis (ALS), or Lou Gehrig’s Disease, is a rare and progressive neurological disorder impacting motor neurons, leading to voluntary muscle function loss in the brain and spinal cord [10]. Despite being more prevalent in individuals aged 40 - 60, ALS can affect younger people, with an annual incidence of 2.6 cases per 100,000 individuals and a higher predisposition in men [11]. ALS manifests through symptoms like muscle weakness, slurred speech, muscle cramps, and breathing difficulties [11]. Diagnosis is challenging due to symptom overlap with other conditions; tests, including EMGs, MRIs, and biopsies, aid in identification [12]. While no cure exists, treatments like Riluzole and Edaravone aim to slow symptom progression [12]. Supportive measures, including respiratory care and therapy, enhance comfort and independence when ALS affects daily functions [12].
Huntingtons disease
Huntington’s disease is a neurological autosomal dominant condition that develops in middle life and is passed down through families from generation to generation. It is marked by dementia, behavioral and psychological disorders, and undesired choreatic motions [13].
The Huntingtin (HTT) gene’s CAG trinucleotide repeats on the short arm of chromosome 4p16.3 is the cause of this condition. This mutation causes the polyglutamine in the HTT protein to expand unusually long, which causes neurodegeneration. Moreover, the enlargement makes the HTT protein more prone to accumulation and aggregation, which hinders protein folding [14]. In HD, the brain generally shrinks, and the striatum (caudate nucleus and putamen) degenerates, specifically losing efferent Medium Spiny Neurons (MSNs). Although the striatum appears to be the most afflicted area of the brain, HD patients have been discovered to have a regionally-specific thinning of the cortical ribbon [15]. The prevalence is equally distributed across men and women globally but has a broad range. According to estimates, there are 5 - 10 instances per 100,000 people, with East Asians having a lower prevalence and white Europeans having a larger one [15]. There is no cure for HD Treatment is mainly pharmacological as well as supportive Many symptomatic treatments are available, and several research are now being conducted in an effort to find a cure for this terrible disease [15].
Alzheimer’s disease (AD)
The most prevalent kind of dementia is a progressive neurological illness most frequently characterized by early memory impairment and cognitive decline that may later impact behavior, speech, visuospatial orientation, and the motor system [16]. Age, genetics, and sex are only a few examples of the variables that affect how long it takes for a continuum of these symptoms to emerge [17]. The Alzheimer’s Association attributes 60–80% of dementia cases to Alzheimer’s disease (AD), a degenerative brain illness [17]. The medial temporal lobe and neocortical regions of the brain, which are the most damaged parts of AD, have amyloid-beta peptide (A beta) buildup that causes neuritis plaques and neurofibrillary tangles [18]. Alzheimer’s Disease (AD) and other brain disorders, infections, abnormalities in the pulmonary and circulatory systems that reduce the amount of oxygen reaching the brain, nutritional deficiencies, vitamin B12 deficiencies, tumors, and other conditions can all contribute to the progressive loss of cognitive abilities [18].
Current therapies for neurodegenerative disorders
Neurodegenerative disorders, encompassing conditions like Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis (ALS), present a formidable challenge to both clinicians and researchers. In this section, we will delve into the current therapies available for these disorders, including pharmacological and non-pharmacological interventions. We will also critically examine their limitations and challenges based on existing research and reviews.
Pharmacological interventions
Alzheimer’s disease:
I. Cholinesterase inhibitors: Current pharmacological interventions for Alzheimer’s disease primarily focus on symptomatic relief. Cholinesterase inhibitors, such as donepezil, rivastigmine, and galantamine, are widely used to enhance cholinergic transmission and temporarily improve cognitive function in patients with mild to moderate Alzheimer’s disease [19]. However, their effects are modest and do not alter the course of the disease. Moreover, adverse effects like gastrointestinal disturbances and bradycardia limit their tolerability [19].
II. NMDA receptor antagonists: Memantine, an NMDA receptor antagonist, is utilized in Alzheimer’s disease management to alleviate cognitive symptoms, with its primary focus being symptomatic relief, potentially offering limited influence on disease progression [20]. The data indicate a slight positive impact of memantine after six months in moderate to severe AD, while its effect on cognition in individuals with mild to moderate vascular dementia remains undetectable in global assessments at the same time frame. The question of whether memantine exerts any discernible effect in mild to moderate AD remains unanswered [20].
Dopaminergic therapy in Parkinson’s disease
Pharmacological management of Parkinson’s disease revolves around enhancing dopaminergic function. Levodopa, in combination with carbidopa, remains the gold standard for motor symptom control [21]. Nevertheless, long-term use can lead to motor fluctuations and dyskinesias. Furthermore, non-motor symptoms, such as cognitive impairment and psychiatric disturbances, often remain unaddressed by these treatments [21].
Riluzole in ALS
Riluzole, an FDA-approved drug for ALS, modestly extends survival by reducing glutamate excitotoxicity [22]. However, its effects are limited, and it does not halt disease progression. The management of ALS largely relies on palliative care and addressing symptomatology [22].
Antipsychotic medications
Behavioural and psychiatric symptoms in neurodegenerative disorders are often treated with antipsychotic medications. Their use is associated with a risk of adverse effects, including sedation and extrapyramidal symptoms [23].
Non-pharmacological interventions
Non-pharmacological interventions play a crucial role in enhancing the quality of life for individuals with neurodegenerative disorders.
1. Physical and occupational therapy: Physical and occupational therapy plays a vital role in preserving functional independence, alleviating motor symptoms, and enhancing overall well-being [24] in individuals with neurodegenerative disorders such as Parkinson’s disease. However, the effectiveness of these therapies may vary among individuals, and access to specialized rehabilitation services can be limited [25].
2. Cognitive training and behavioural interventions: Cognitive training programs, such as memory training and cognitive stimulation, have been developed to enhance cognitive functions in patients with Alzheimer’s disease [26]. While these interventions show promise in improving cognitive performance, their long-term efficacy remains uncertain, and they are typically used alongside pharmacological treatments. This non-pharmacological approach also aids in managing mood and behavioural symptoms in neurodegenerative disorders and may be influenced by factors such as the patient’s willingness to engage in therapy and the presence of cognitive impairment [27].
Limitations and challenges
Despite these therapeutic options, neurodegenerative disorders continue to impose significant burdens on patients and their caregivers. Several limitations and challenges are evident in the current treatment landscape:
1. Limited disease modification: The available pharmacological treatments for neurodegenerative disorders primarily offer symptomatic relief and do not address the underlying disease pathology [19,21].
2. Adverse effects: Many pharmacological interventions are associated with adverse effects, which can limit their long-term use and tolerability [19,21].
3. Non-motor symptoms: Non-motor symptoms, such as cognitive impairment and psychiatric disturbances, often remain undertreated in neurodegenerative disorders [21].
4. Lack of disease-modifying drugs: Despite extensive research efforts, there are currently no disease-modifying drugs available for ALS [22].
5. Access to rehabilitation services: Access to specialized rehabilitation services, including physical and occupational therapy, is unevenly distributed, limiting the benefits that some patients can receive [24].
6. High healthcare costs: The cost of long-term care for individuals with neurodegenerative disorders can be financially burdensome for both patients and healthcare systems [26].
In conclusion, the current therapies for neurodegenerative disorders, while offering some relief, are far from ideal. They primarily address symptoms and come with limitations and challenges related to efficacy, tolerability, and accessibility (Table 1). Emerging therapies, such as gene therapy and stem cell treatments, hold promise in addressing some of these limitations, as discussed in subsequent sections.
| Table 1: Currently available therapy options for neurodegenerative diseases. | |||
| Neurological Disorder | Class | Drugs | Mechanism of Action |
| Alzheimer's Disease | Acetylcholinesterase Inhibitors | Donepezil, Rivastigmine, Galantamine | Enhance cholinergic neurotransmission by inhibiting acetylcholinesterase, thus increasing acetylcholine levels in the brain. |
| Monoclonal antibody (mAb) | Aducanumab | It targets and eliminates amyloid-beta plaques | |
| NMDA Receptor Antagonists | Memantine | Modulate glutamate signalling by blocking NMDA receptors, which may reduce excitotoxicity and cognitive decline. | |
| Parkinson's Disease | Dopaminergic Medications | Levodopa (often in combination with Carbidopa) | Converts to dopamine in the brain, replenishing dopamine levels in the basal ganglia to alleviate motor symptoms. |
| Dopaminergic Agonists | Ropinirole, Pramipexole | Directly stimulate dopamine receptors to mimic the effects of dopamine in the brain. | |
| ALS (Amyotrophic Lateral Sclerosis) | Glutamate Modulator | Riluzole | Modulates glutamate neurotransmission, potentially slowing disease progression. |
| Huntington's Disease | Vesicular Monoamine Transporter Inhibitor | Tetrabenazine | Inhibits the vesicular monoamine transporter (VMAT2), reducing the release of dopamine and other monoamines, thus mitigating chorea and motor symptoms. |
| Antipsychotic Medications | Haloperidol, Quetiapine | Manage psychiatric and behavioural symptoms by blocking dopamine and other neurotransmitters. | |
Emerging therapy
Stem cell therapy for neurodegenerative disorders: Stem cell therapy stands as a promising approach for treating neurodegenerative diseases. Stem cells play multiple roles, acting as immunomodulators and neuroprotectors, and possessing the capacity to replace lost neurons and integrate into the nervous system. This therapy can be utilized to deliver therapeutic factors and potentially impede disease progression. Both preclinical and clinical studies have demonstrated beneficial outcomes for stem cell therapy, showing promise across various forms of neurodegenerative disorders. Ongoing and future clinical trials are expected to provide crucial insights into aspects such as immunosuppression, graft survival, and the overall efficacy of stem cell therapy. In essence, stem cell therapy holds significant hope for the treatment of a wide range of neurodegenerative diseases [28-30].
Types of stem cells that have been considered for use in treating neurodegenerative diseases
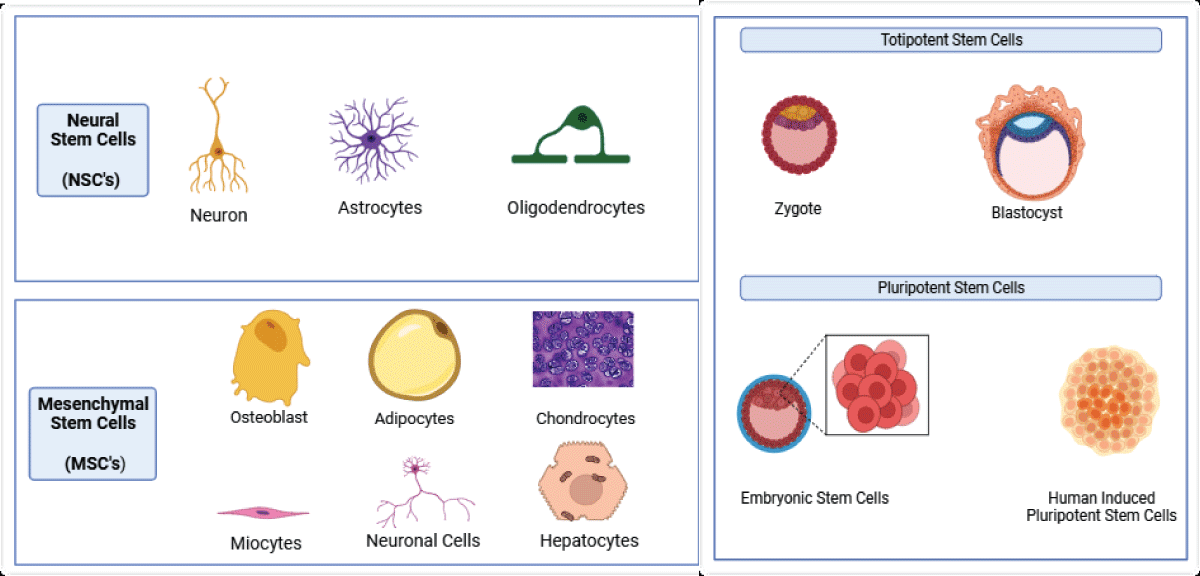
Several types of stem cells have been considered for use in treating neurodegenerative diseases, each with its own characteristics (Figure 1). These include:
1. Embryonic Stem Cells (ESCs): Derived from embryos, these cells have the potential to differentiate into various cell types, including neurons. However, their use is controversial due to concerns about teratoma formation and the ethical considerations associated with the destruction of human embryos [28,31].
2. Induced Pluripotent Stem Cells (iPSCs): Generated by reprogramming adult somatic cells to exhibit pluripotent properties, iPSCs offer an alternative to ESCs, addressing ethical concerns. However, their efficiency and safety in the treatment of neurodegenerative diseases are still under investigation [28,31].
3. Mesenchymal Stem Cells (MSCs): Derived from the stromal compartment of the bone marrow, MSCs can differentiate into various cell types, including neurons. They possess immunomodulatory and neuroprotective properties, making them a promising option for treating neurodegenerative diseases [28,29].
4. Neural Stem Cells (NSCs): Derived from the Subventricular Zone (SVZ) and Subgranular Zone (SGZ) of the brain, NSCs can differentiate into various cell types, including neurons and glial cells. They show potential in treating neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease [32].
Figure 1: Types of Stem cells commonly used.
The choice of stem cell type for treatment depends on the specific neurodegenerative disease and the patient’s condition, considering the advantages and limitations of each type. Ongoing and future clinical trials will provide more insights into the efficacy and safety of these stem cell types in treating neurodegenerative diseases.
Mechanism of stem cell therapy in neurodegenerative disorders
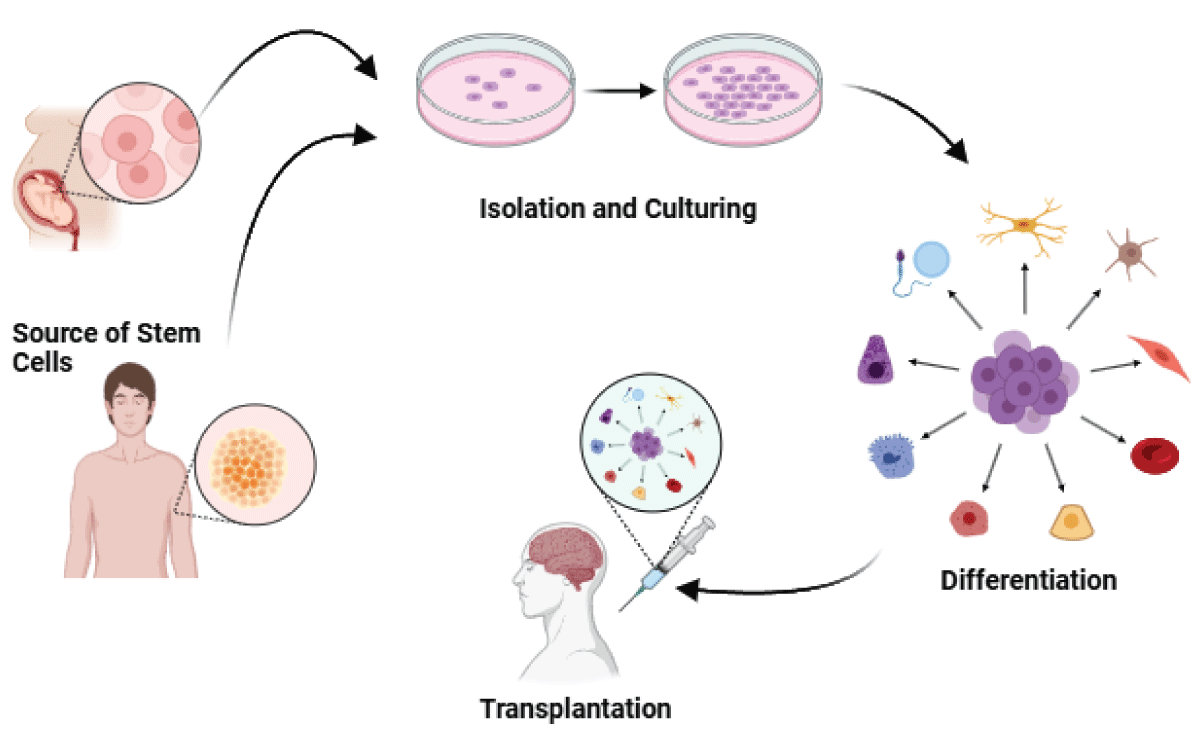
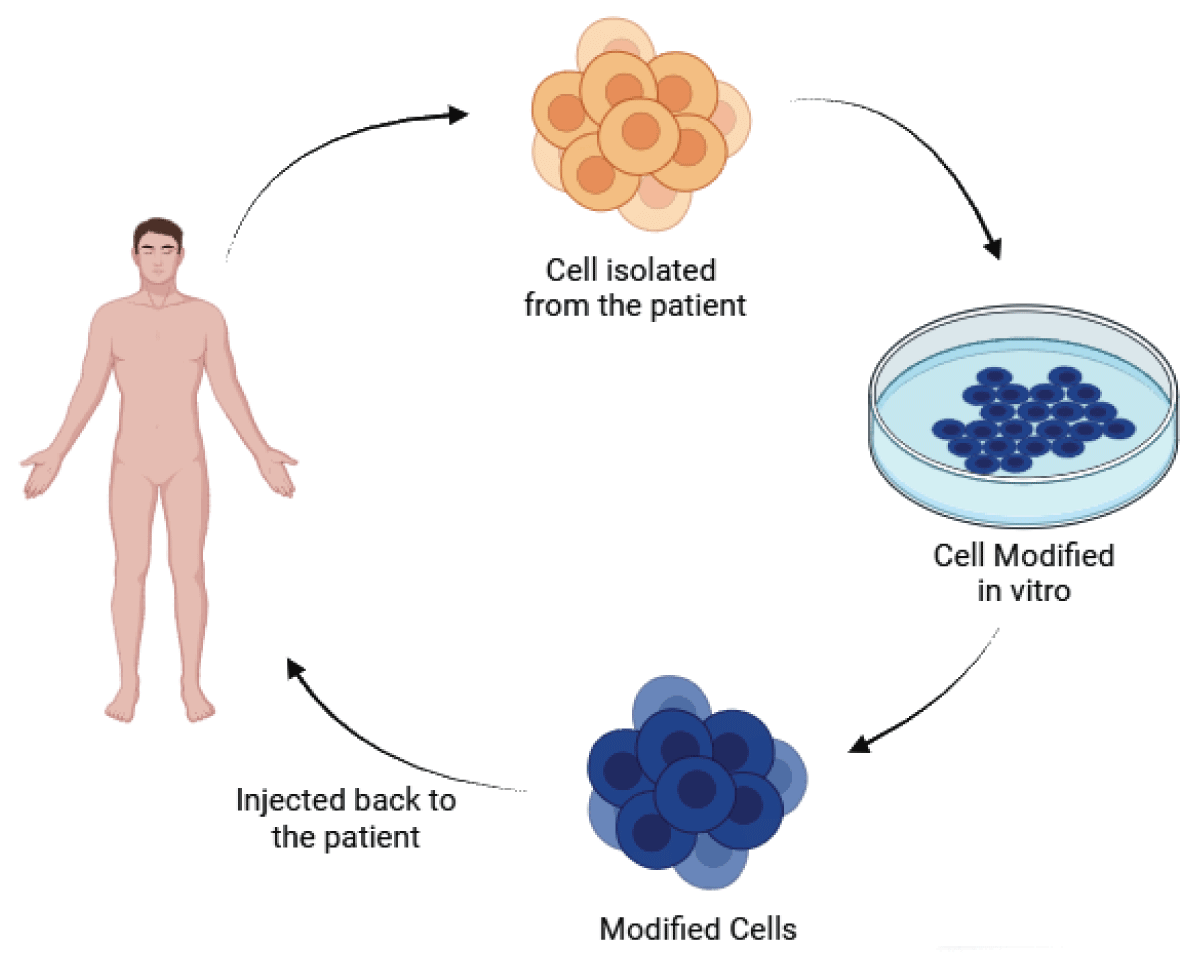
Stem cell therapy has demonstrated promise in the treatment of neurodegenerative disorders, including Lysosomal Storage Diseases (LSDs) and other conditions characterized by the progressive loss of neurons and cognitive function [33,34]. The mechanisms of stem cell therapy in neurodegenerative disorders (Figure 2) encompass the following:
1. Replacing Damaged Cells: Stem cells contribute to the repair of injured neuronal tissue by replacing damaged or lost cells with differentiated cells, aiding in the restoration of the structure and function of affected brain regions [34].
2. Creating a Conducive Environment: Stem cells can establish a supportive environment that favors regeneration, promoting the growth of healthy neurons and shielding them from further damage [34].
3. Neurotrophic Support: Stem cells release growth factors and other proteins that provide neurotrophic support, aiding in the growth and survival of neurons. This, in turn, can improve cognitive function and alleviate symptoms of neurodegenerative disorders [34].
4. Immunomodulation and Enhancement of Endogenous Repair Mechanisms: Transplanted neural stem/precursor cells can protect the injured central nervous system through mechanisms such as immunomodulation and enhancement of the body’s natural repair processes [35].
5. Cell Replacement: In certain cases, stem cell therapy involves directly replacing damaged neurons with healthy ones, contributing to the restoration of brain function and the alleviation of symptoms in neurodegenerative disorders [35].
Figure 2: Flow of Stem Cell Therapy.
Despite promising preclinical results, the clinical applications of stem cell therapy for neurodegenerative disorders remain limited. Further research and development are necessary to enhance treatment outcomes and achieve complete recovery for patients [33]. Combining stem cell therapy with other innovative approaches, such as nanoparticle technology, may further enhance the effectiveness of treatments for neurodegenerative disorders [36].
Potential benefits of stem cell therapy for neurodegenerative disorders
There are ongoing clinical trials exploring the potential benefits of stem cell therapy in various neurodegenerative disorders. Here are some examples of current clinical trials:
1. MSCs in Parkinson’s disease: A clinical trial is assessing the safety and efficacy of intravenously administered allogeneic Mesenchymal Stem Cells (MSCs) in patients with Parkinson’s disease. The trial is sponsored by TCT2004 and registered under the identifier NCT0412644 [37].
2. MSCs in Amyotrophic Lateral Sclerosis (ALS): An ongoing phase I clinical trial is investigating the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary efficacy of intravenous administration of autologous bone marrow-derived MSCs in patients with ALS. The trial is sponsored by Biostar and registered under the identifier NCT0328346 [28].
3. NSCs in stroke: A clinical trial is evaluating the safety and efficacy of intracerebral transplantation of human Neural Stem Cells (NSCs) in patients with stroke. The trial is sponsored by Lifespan Brain Health and registered under the identifier NCT0330848 [38].
4. iPSCs in stroke: An ongoing phase I clinical trial is investigating the safety, tolerability, pharmacokinetics, and pharmacodynamics of intravenous administration of autologous induced Pluripotent Stem Cells (iPSCs) in patients with stroke. The trial is sponsored by Stem Cells Inc. and registered under the identifier NCT0355484 [39].
These clinical trials aim to assess the safety and efficacy of stem cell therapy in treating various neurodegenerative diseases. It’s important to note that the outcomes of these trials may vary, and further research is needed to determine the optimal stem cell type and delivery method for each specific disease.
Advantages and disadvantages
Stem cell therapy holds great promise for the treatment of neurodegenerative disorders, However, like any medical treatment, it has both advantages and disadvantages.
Advantages of stem cell therapy
1. Cell replacement and repair: Stem cells have the potential to replace damaged or lost cells, particularly beneficial for neurodegenerative diseases characterized by specific types of neuron loss [40].
2. Neurotrophic support: Stem cells can secrete neurotrophic and growth factors critical for the growth, survival, and differentiation of developing neurons [41].
3. Decreased neuroinflammation: Stem cells may help decrease neuroinflammation, a common characteristic of neurodegenerative diseases [40].
4. Activation of endogenous stem cells: Stem cells can activate endogenous stem cells in the body, potentially aiding in repair and regeneration [40].
Disadvantages of stem cell therapy
1. Ethical concerns: The use of certain stem cell types, particularly embryonic stem cells, raises ethical issues due to the destruction of human embryos [42].
2. Immune rejection: There’s a risk of immune rejection, especially with the use of non-autologous stem cells [43].
3. Risk of tumor formation: The high proliferation rate of stem cells poses a risk of tumor formation [43].
4. Treatment-related mortality: Some stem cell therapies, like Autologous Haematopoietic Stem Cell Transplantation (AHSCT), have had substantial treatment-related mortality [29].
It’s crucial to note that while stem cell therapy holds promise for treating neurodegenerative disorders, it is still in the experimental stage. Further research is needed to fully understand the potential benefits and risks.
Potential risks and challenges of stem cell therapy for neurodegenerative disorders
Stem cell therapy, while promising, does come with potential risks and side effects, particularly when used for the treatment of neurodegenerative disorders.
1. Limited clinical application challenges:
- Short half-life and rapid clearance: Stem cell therapy faces challenges related to the short half-life, limited targeting, and rapid clearance of stem cells after application [44].
- Insufficient payload: The therapy may be limited by challenges in delivering a sufficient payload to the target site [44].
2. Safety concerns:
- Risk of tumorigenesis: There is a potential risk of tumor formation, or tumorigenesis, associated with stem cell transplantation therapy [44].
- Detrimental effect on microenvironment: Stem cell therapy may have a detrimental effect on the microenvironment, influencing the surrounding tissues [44].
3. Challenges in stem cell survival and migration:
- Low efficiency of survival and migration: The efficiency of survival and migration of neural stem cells into the brain is a challenge in cell-based therapy [42].
4. Optimization strategies:
- ROCK inhibitors: The use of Rho-associated kinases (ROCKs) inhibitors, such as fasudil, is suggested to optimize cell therapy by increasing the activity of neural stem cells and mesenchymal stem cells, inhibiting inflammatory responses, and promoting the production of neurotrophic factors [42].
5. Research needs:
- Ongoing research: Further research is needed to address challenges related to safety, potency, genetic stability, immunogenicity, tumorigenicity, cell reproducibility, scalability, and engraftment [44].
Despite these challenges, ongoing research and optimization strategies, such as the use of ROCK inhibitors, aim to enhance the safety and efficacy of stem cell therapy for neurodegenerative disorders [42].
Ongoing clinical trials for stem cell-based therapies for neurodegenerative disorders
There are several ongoing clinical trials for stem cell-based therapies for neurodegenerative disorders. Some of these trials include:
1. Mesenchymal stem cell-based therapy for Lysosomal Storage Diseases (LSDs) and other neurodegenerative disorders:
- Investigates the use of mesenchymal stem cells for treating Lysosomal Storage Diseases (LSDs) and other neurodegenerative disorders.
- Focuses on central nervous system neuro degeneration [33].
2. Nanoparticle technology and stem cell therapy:
- Explores the combination of nanoparticle tech-nology and stem cell therapy to enhance the effectiveness of treatments for neurodegenerative disorders [36].
3. Neural stem cell therapy for neurodegenerative disorders:
- Examines the role of neurotrophic support in neural stem cell therapy for treating neurodegenerative disorders.
- Focuses on addressing the progressive loss of structure and/or function of neurons [34].
4. Regenerative stem cell therapy for neuro-degenerative diseases:
- Provides an overview of various types of stem cells.
- Discusses current knowledge of stem cell-based therapies in neurodegenerative diseases and recent advances in the field.
- Highlights the potential of stem cells to repair injured neuronal tissue, create a conducive environment for regeneration, and protect healthy neurons and glial cells [34].
These clinical trials and studies signify a growing interest in stem cell-based therapies for neurodegenerative disorders. While promising, it is crucial to emphasize that the safety and efficacy of these therapies are still under investigation, and further research is needed to confirm their potential benefits for patients.
Gene therapy for neurodegenerative diseases
Gene therapy holds significant promise in offering therapeutic benefits to millions of individuals grappling with neurodegenerative diseases such as Parkinson’s, Huntington’s, and Alzheimer’s. The potential avenues for this therapy include directly correcting pathogenic mechanisms, providing neuroprotection, facilitating neuro-restoration, and managing symptoms [45]. However, the effectiveness of these therapeutic interventions hinges on a deep understanding of the underlying disease pathogenesis and the precise temporal and spatial specificity of gene expression [45].
Mechanism of gene therapy
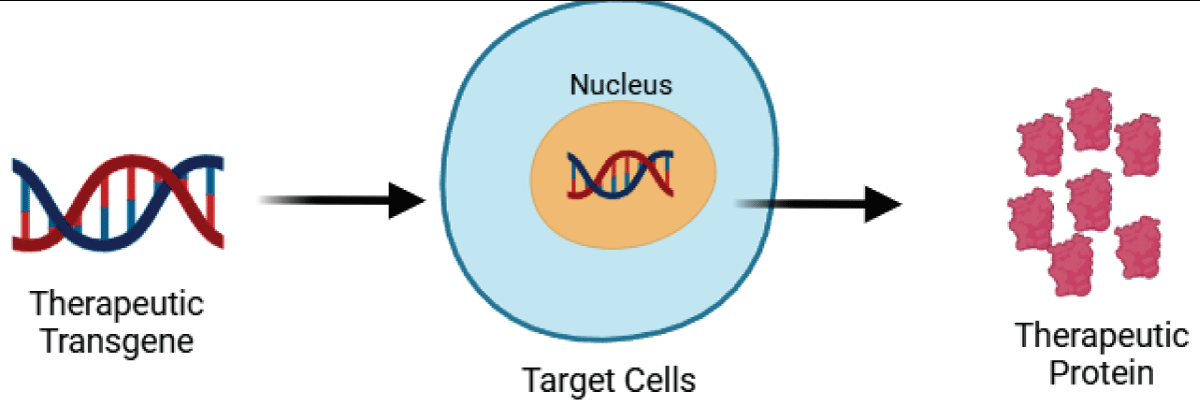
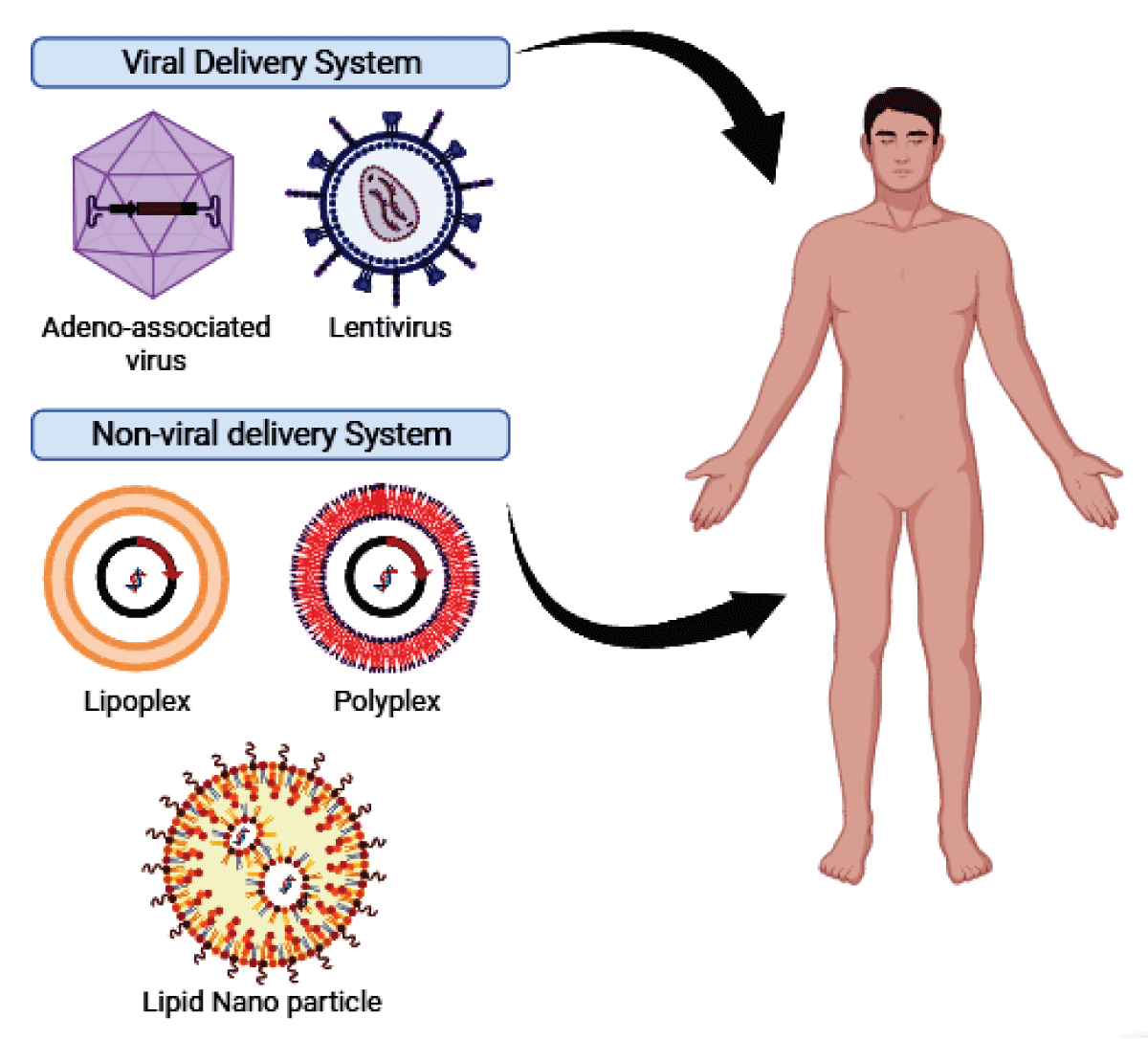
Gene therapy involves intentionally modifying gene expression in specific cells to address pathological conditions by introducing exogenous nucleic acids (Figure 3). The delivery of these nucleic acids is typically facilitated by carriers or vectors, with viral vectors and non-viral vectors being the two main types. (Figure 4).
Figure 3: Gene Therapy Concept.
Figure 4: Types of Vectors used in Gene therapy.
Viral vectors, such as Adeno-Associated Virus (AAV) vectors, show significant potential in treating genetic disorders. However, they may lead to Infusion-Associated Reactions (IARs), posing a substantial challenge to the safety and efficacy of gene therapy. IARs are linked to toll-like receptor activation, pro-inflammatory cytokine production, and the generation of neutralizing antibodies and T-cell responses that can interfere with gene therapy [46].
Non-viral vectors, encompassing polymers, lipids, and inorganic particles, have been a rapidly advancing area in gene delivery research. They exhibit low cytotoxicity, immunogenicity, and mutagenesis, making them an appealing option for gene therapy. Nevertheless, they face challenges like gene transfer efficiency, specificity, gene expression duration, and safety [47].
The mechanisms of gene therapy involve delivering exogenous nucleic acids into target cells, their uptake, intracellular trafficking, and expression. Both viral and non-viral vectors have specific mechanisms to facilitate the movement of DNA complexes through cells. Understanding and leveraging these mechanisms are critical for the success of gene therapy [48].
In summary, gene therapy mechanisms encompass delivering and expressing exogenous nucleic acids in target cells, achievable through viral or non-viral vectors, each with its own advantages and challenges. Ongoing research focuses on enhancing the safety, efficacy, and specificity of gene therapy using both types of vectors.
Types of gene therapy
Gene therapy encompasses various approaches, each designed to address specific genetic conditions with their own set of advantages and challenges (Figure 5). Here are some main types of gene therapy:
1. Gene addition therapy: This method involves introducing a normal copy of a gene into cells to compensate for a defective gene. It is commonly used for genetic disorders caused by single-gene mutations [49].
2. Gene editing: Gene editing enables the modification of specific genes within a cell. It can correct mutations, insert new genes, or edit existing genes to address genetic disorders [49].
3. Gene silencing: This technique employs small interfering RNAs (siRNAs) to suppress the expression of specific genes. It is useful for treating genetic disorders caused by the overexpression of certain genes [49].
4. Gene correction therapy: This approach aims to correct the mutations responsible for a genetic disorder. It may involve the use of enzymes like CRISPR-Cas9 to edit the DNA within a cell, rectifying the mutations causing the disorder [49].
Figure 5: Gene Therapy Process.
Various gene therapy approaches are in development and testing for a range of diseases, including inherited disorders, certain types of cancer, and specific viral infections [49]. The choice of a gene therapy approach depends on factors such as the specific disease, underlying genetic mutations, and the desired therapeutic outcomes. Ongoing research is dedicated to enhancing the safety, efficacy, and specificity of gene therapy for diverse diseases and conditions [49-51].
Challenges in developing gene therapy for genetic disorders
The development of gene therapy for genetic disorders encounters several challenges, including:
1. Delivery methods: Ensuring the efficient and targeted delivery of therapeutic genes to the relevant cells or tissues without causing off-target effects or triggering immune responses is a significant challenge [52].
2. Safety and efficacy: Overcoming safety concerns, such as the risk of immunogenic reactions, off-target effects, and the potential for long-term adverse events. Additionally, ensuring sustained and effective expression of the therapeutic gene poses a critical challenge [52,53].
3. Regulatory hurdles: Navigating the complex regulatory landscape to obtain approval for gene therapy treatments, which involves demonstrating safety, and efficacy, and providing long-term follow-up data for patients [54].
4. Specificity and control: Achieving precise control over gene expression and avoiding unintended consequences, such as the development of cancer or other unexpected outcomes, is a crucial challenge [55].
5. Vector development: Optimizing the design and production of viral and non-viral vectors for gene delivery, including enhancing their specificity, reducing immunogenicity, and increasing their capacity for gene transfer, presents a significant challenge [54,56].
6. Biomarker development: Developing effective biomarkers to assess the effectiveness of gene therapy and monitor long-term outcomes is an essential aspect of addressing challenges in this field [52].
Addressing these challenges is vital for the successful development and widespread application of gene therapy for genetic disorders. Ongoing research and technological advancements are focused on overcoming these hurdles to improve the safety, efficacy, and specificity of gene therapy.
Potential benefits of gene therapy
Gene therapy holds significant potential for providing substantial benefits in the treatment of neurodegenerative diseases. Some of these potential advantages include:
1. Permanent cure: Gene therapy aims to target the underlying genetic mutations or defects responsible for neurodegenerative diseases, offering the potential for a permanent cure for these conditions [52].
2. Improved quality of life: By addressing the root cause of neurodegenerative diseases, gene therapy has the potential to slow down or halt disease progression, leading to an improved quality of life for patients [52].
3. Reduced healthcare costs: A permanent cure for neurodegenerative diseases could significantly reduce healthcare costs associated with the chronic treatment and management of these conditions [52].
4. Customized treatments: Gene therapy can be tailored to the specific needs of each patient, considering their unique genetic makeup and the severity of their disease [52].
5. Reduced burden on caregivers and families: Gene therapy has the potential to alleviate the burden on caregivers and families by providing long-term solutions for patients with neurodegenerative diseases [52].
6. Advancements in delivery methods: Ongoing research is focusing on improving gene delivery methods, such as viral and non-viral vectors, to enhance the effectiveness and safety of gene therapy for neurodegenerative diseases [52,57].
Despite these promising benefits, gene therapy also faces challenges, including safety concerns, regulatory hurdles, and the need for effective biomarkers to assess treatment outcomes [52,54]. Nevertheless, the increasing research and clinical trials in this field are expected to address these challenges and further advance the application of gene therapy for neurodegenerative diseases.
Potential risk of gene therapy in neurodegenerative diseases
The potential risks associated with gene therapy for neurodegenerative diseases include:
1. Immunogenic reactions: Both viral and non-viral vectors utilized in gene therapy may elicit immune responses, leading to inflammation and potential tissue damage [52,58].
2. Off-target effects: There is a risk that the introduced genes may have unintended effects on non-target tissues or genes, potentially resulting in adverse events or complications [58].
3. Lack of effective biomarkers: The absence of reliable biomarkers to assess the effectiveness of gene therapy and monitor long-term outcomes poses a challenge in evaluating the safety and efficacy of these treatments [52].
4. Long-term safety concerns: The long-term safety of gene therapy for neurodegenerative diseases is not yet fully understood, and there is a risk of unforeseen adverse events, such as the development of cancer or other complications [58].
5. Delivery challenges: Ensuring precise and efficient delivery of therapeutic genes to specific regions of the brain without causing damage or off-target effects remains a significant challenge in gene therapy for neurodegenerative diseases [58].
Addressing these risks is critical for the safe and effective application of gene therapy for neurodegenerative diseases. Ongoing research is focused on optimizing gene delivery methods, enhancing the safety of vectors, and developing effective monitoring strategies to mitigate these potential risks [52,58].
Recent development in gene therapy
Recent developments in gene therapy for neurodegenerative diseases have been marked by a focus on clinical trials and emerging concepts. In the context of Parkinson’s Disease (PD), various gene therapy treatment strategies have been developed and assessed in patients. These strategies encompass the enhancement of dopamine synthesis, expression of trophic factors, and neuromodulation. Genes are typically delivered via viral vectors and expressed within neurons in PD-relevant areas of the brain, such as the striatum. While current gene therapy strategies do not address non-motor symptoms of PD and do not curtail α-synuclein aggregation/spread, they have demonstrated safety in clinical trials, and ongoing studies aim to explore their efficacy [59].
In the field of inherited retinal degeneration, adeno-associated virus (AAV)-based gene therapies have made significant progress, with a focus on reducing vision loss and associated morbidities. The recent approval of Luxturna® (voretigene neparvovec-rzyl) for Leber congenital amaurosis type 2 (LCA2) has prompted a review of current clinical trials for inherited retinal diseases, underscoring the importance of AAV-based gene therapies [60].
While some clinical studies have not demonstrated therapeutic efficacy, the use of AAV as a delivery tool has been shown to be safe [61]. Moreover, RNAi-based gene therapies have been extensively studied in preclinical models and have recently advanced to clinical studies, representing a newly emerging approach in the field [61].
Overall, gene therapy for neurodegenerative diseases is an evolving field, with ongoing clinical trials and research dedicated to improving the safety and efficacy of these innovative treatments.
Immunotherapy
Neurodegenerative diseases are a global health concern due to increased longevity, but no disease-modifying treatments exist. Direct immunotherapy targets misfolded proteins in brains to slow or prevent disease expression [62].
Immunotherapy targets protein aggregates in membrane and intracellular spaces, blocking propagation, triggering lysosomal clearance, reducing inflammation, and promoting neuroprotection through various mechanisms [63].
Immunotherapy aims to reduce protein accumulation by neutralising toxic species. The first trial against Aβ revolutionized vaccine design, shifting from active to passive immunization. Neurodegenerative diseases, like Alzheimer’s, involve abnormal protein aggregation, with most immunotherapies targeting Aβ. The field has also targeted α-syn [64].
There are two types of immunotherapies Active and passive
Active Immunisation
Active vaccination involves the introduction of an exogenous substance to stimulate the immune system to initiate a response [65].
Amyloid-β targeting therapies include AN-1792, ACI-24, CAD106, ACC-001, and ABvac40. AN-1792 cleared Aβ40, Aβ42, and Aβ43 from AD plaques. Other vaccines include liposome-based, anti-Aβ, and N-terminus peptide-based ones [66].
Passive Immunisation
Passive vaccination involves directly introducing exogenous antibodies into an animal or person, providing a similar benefit as active vaccination [65].
PD treatment involves vaccines and passive immunotherapies targeting extracellular alpha-synuclein binding and Fcγ effector function, primarily through microglial-mediated degradation [67].
Recent studies show that immunotherapeutic approaches to neurodegenerative illnesses, such as Alzheimer’s and Parkinson’s diseases, can reduce the aggregation of polyglutamine repeat sequences in COS-7 cells, providing hope for introducing endogenous antibody expression in HD and other neurodegenerative diseases [68].
Neurotropic factors
Neurotrophic factors, also known as neurotrophins, are growth factors that regulate differentiation and support growth in the vertebrate nervous system. They consist of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and neurotrophin 4 (NT4). These factors bind to tyrosine receptor kinase (Trk) family members to elicit survival responses. The balance between neuronal survival and death depends on the level of neurotrophin and the type of receptors. Neurotrophins are essential for memory acquisition, retention, long-term potentiation, and cholinergic innervation throughout adulthood [69].
Neurotrophic factors (NTFs) have been found to support neuronal survival and function in the adult central nervous system, leading to interest in their potential intervention in neurodegenerative diseases [70].
Neurotrophins are precursor proteins cleaved at dibasic amino acids to produce mature 118 - 130 amino acids. They are noncovalent dimers of 13 kDa polypeptide chains bound together by disulfide bridges in a cysteine knot motif and held together by hydrophobic interactions. Neurotrophins include CDNF, MAF, BDNF, NT4, NT3, NGF, and GDNF, which are essential for maintaining healthy brain function and growth [71].
Neurotrophins, including brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), share a common ancestral gene and similar structure [72].
Compounds targeting selective neuron types are promising therapeutic agents for neurodegenerative disorders like amyotrophic lateral sclerosis, brain-derived neurotrophic factor, Alzheimer’s disease, peripheral neuropathy, and Parkinson’s disease, as they have been used in humans [73].
Glial Cell-Derived Neurotrophic Factor (GDNF) is a neurotrophic factor with a high specificity for dopaminergic neurons, making it a potential therapy for neurodegenerative diseases like Parkinson’s disease (PD). It has also shown trophic and protective effects on noradrenergic neurons in the locus coeruleus and peripheral motor neurons, providing hope for its therapeutic potential in HD and ALS. However, the therapeutic mechanism of action of GDNF is not fully defined, and the degenerating brain of PD may be resistant to its neuroprotective potential. Ciliary Neurotrophic Factor (CNTF) is another interesting NTF with potent effects on the development and maintenance of the nervous system, cardiomyocytes, osteoblasts, immune cells, adipocytes, and skeletal muscle cells. CNTF has been found to affect motor neuron survival in vitro, during development, after injury to motor neuron systems, and in genetic models of motor neuron degeneration [72].
Various NTF delivery vectors and systems have been applied to deliver therapeutics into the central nervous system (CNS) with promising results. Stem cells have a superior advantage due to their lack of cytotoxic concerns and transgene size limitations, while viral vectors have a low risk of tumour and glial scar formation. Combining NTF-loaded biomaterial systems with natural and synthetic substances is more efficient. Future studies should focus on improving stem cell-based vectors and viral vectors and optimizing cellular, viral vectors, and biomaterial systems to provide standards for clinical applications.
RNAi (RNA interference)
RNA interference (RNAi) is a naturally occurring, evolutionarily conserved biological process that controls gene expression and acts as an inherent defensive mechanism against transposable elements and viral invasion [74]. RNA interference (RNAi) is a new gene regulation mechanism that suppresses transcription (transcriptional gene silencing) or triggers a sequence-specific RNA degradation process (posttranscriptional gene silence) to reduce the number of transcripts produced [75]. A condensed model of the RNAi pathway has two phases and three essential components in common: I dsRNA inducers, (ii) homology-dependent RNA target degradation, and (iii) the presence of proteins that are particularly engaged in the degradative machinery [75].
By processing double-stranded RNA (dsRNA) into useful short RNAs (21 nt), RNAi enables sequence-specific gene silencing [74]. Short interfering RNAs (siRNAs) and microRNAs (miRNAs), which have different cellular biogenesis but converge into the same molecular route for mediating mRNA cleavage, are two kinds of small RNAs that mediate RNAi [76]. The first stage entails the binding of RNA nucleases to a large dsRNA and the fragmentation of it into RNAs of 21 - 25 nucleotides (siRNA) [77] Double-stranded RNAs with a length of 21 to 25 nucleotides and 2-nucleotide overhands on each strand are known as siRNAs. Simian RNAs (siRNAs), short hairpin RNAs (shRNAs), and primary miRNA precursors are examples of potential therapies that take advantage of this naturally occurring process [76]. RNase III contributes to the start of RNAi [77].
With 3-overhangs of 2 to 3 nucleotides, 5-phosphate, and 3-hydroxyl termini, RNAIII, also known as Dicer, breaks down dsRNA into homogeneously siRNA pieces [77]. Dicer is a protein whose last 110 amino acids are included in the RDE1/QDE2/Argonaute family of proteins, which has been genetically related to RNAi [77].
The active RNAi effector complex, also known as RISC, is formed when Ago2 has just a single guide strand, having already discarded the cleaved passenger strand. On the miRNA route, RISC performs the last step in gene silencing. The “slicer” or catalytic component of Ago2 cleaves the target mRNA, causing a decrease in the target mRNA level and a subsequent decrease in the target protein level.
The biological process of RNAi in the case of miRNAs starts with the nuclear transcription of lengthy primary miRNA precursors that comprise stem-loop structures containing the miRNAs [77]. Pri-miRNA is identified by a complex produced by DGCR8 and Drosha. In contrast to Drosha, an RNAase III ribonuclease that cleaves the pri-miRNA to produce the 70-nucleotide long hairpin RNA known as miRNA precursors, DGCR8 is a dsRNA binding protein that aids in determining the cleavage location (pre-miRNA) Whereas the RNAi-induced silencing complex (RISC) cleaves target mRNAs, Drosha and Dicer are parts of protein complexes involved in processing dsRNAs [75].
Depending on the degree of sequence complementarity, siRNA can either translationally suppress or degrade its target. Although translational suppression happens when short RNAs bind to target mRNAs insufficiently, transcript breakdown normally necessitates a high degree of complementarity (traditionally in the 30 UTR) [75].
The mRNA or protein target is often only partially eliminated by further post-transcriptional silencing by siRNAs, for instance between 30 and 50% [76].
RNAi in AD (Alzheimer’s Disease)
Tau protein-based neurofibrillary tangles and the accumulation of amyloid (A) peptides represent pivotal features of Alzheimer’s Disease (AD). It is postulated that these deposits give rise to neurotoxicity and brain inflammation, leading to the clinical manifestations observed in individuals with AD. Consequently, the primary objective in AD treatment development revolves around reducing the synthesis of A peptide [74].
RNAi in PD (Parkinson’s Disease)
In Parkinson’s Disease (PD), an abnormal process of protein clearance leads to the buildup of cellular proteins. Within the dopaminergic neurons of the substantia nigra, where most neurodegeneration occurs, distinctive intra-cytoplasmic aggregates known as Lewy bodies form. A key constituent of these Lewy bodies is synuclein. When synuclein is excessively expressed or altered (as observed in A53T and A30P mutations linked to familial PD), this protein prone to aggregation becomes hazardous. Consequently, researchers are focusing on utilizing RNA interference (RNAi) to suppress synuclein as a potential therapeutic target. Some forms of PD without apparent genetic components lack the presence of RNAi. For instance, enhancing the lifespan of dopaminergic neurons may be achieved by reducing proapoptotic markers associated with PD pathogenesis through RNA interference (RNAi) [74].
RNAi in HD (Huntington’s Disease)
Huntington’s Disease (HD) presents a critical challenge that requires attention. Huntingtin, a product of the HD gene, plays a crucial role in brain development. Completely suppressing both the normal and mutant HD genes may inflict significant damage on the adult brain. Thus, there is a need to develop allele-specific silencing techniques that selectively target the mutant allele while sparing the normal one. The optimal solution for HD remains uncertain, but research from various laboratories, including ours, has demonstrated the feasibility of allele-specific silencing, even when the disease and normal alleles differ by a single nucleotide [76]. Huntingtin has been associated with various functions, including vesicle transport, NMDA receptor modulation, and transcriptional control. Given the critical role of huntingtin in essential developmental and cellular processes, controlled and regulated therapeutic RNAi expression methods may be necessary to ensure patient safety in clinical applications. Furthermore, in cases where the loss of wild-type huntingtin is not tolerable in adult neurons, precise suppression of mutant allele production could mitigate adverse effects [74].
In certain disorders, achieving a partial reduction of the molecular target is not only sufficient for normalizing pathology but also desirable, as it allows for the retention of some protein production to support regular cellular function. However, for some disorders, complete elimination of the molecular target from every aberrant cell may be necessary, and this can be challenging with current RNAi delivery methods [76].Neurodegenerative diseases present a formidable global health challenge, impacting millions worldwide. Current therapeutic interventions predominantly focus on symptomatic relief and fall short of addressing the root causes of these conditions. Enter the realm of emerging therapies—stem cell therapy, gene therapy, immunotherapy, and neurotrophic factors—which hold great promise in reshaping the landscape of neurodegenerative disorder treatment. These innovative approaches target pathological processes, providing avenues for neuroprotection and facilitating neurorestoration. However, the journey towards effective treatment is not without its hurdles. Delivery methods, safety concerns, regulatory intricacies, and the development of reliable biomarkers pose significant challenges to these therapies. The ongoing commitment to research and clinical trials is geared towards surmounting these obstacles, with the ultimate goal of enhancing the safety and efficacy of these groundbreaking treatments. The future of neurodegenerative disease treatment lies in the development of innovative and personalized approaches. These approaches are envisioned not only to provide immediate relief but also to yield sustained, long-term benefits, ultimately improving the quality of life for patients and their dedicated caregivers.
- Wilson DM 3rd, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023 Feb 16;186(4):693-714. doi: 10.1016/j.cell.2022.12.032. PMID: 36803602.
- Merelli A, Czornyj L, Lazarowski A. Erythropoietin: a neuroprotective agent in cerebral hypoxia, neurodegeneration, and epilepsy. Curr Pharm Des. 2013;19(38):6791-801. doi: 10.2174/1381612811319380011. PMID: 23530506.
- Melo A, Monteiro L, Lima RM, Oliveira DM, Cerqueira MD, El-Bachá RS. Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxid Med Cell Longev. 2011;2011:467180. doi: 10.1155/2011/467180. Epub 2011 Nov 24. PMID: 22191013; PMCID: PMC3236428.
- Lamptey RNL, Chaulagain B, Trivedi R, Gothwal A, Layek B, Singh J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int J Mol Sci. 2022 Feb 6;23(3):1851. doi: 10.3390/ijms23031851. PMID: 35163773; PMCID: PMC8837071.
- Ding C, Wu Y, Chen X, Chen Y, Wu Z, Lin Z, Kang D, Fang W, Chen F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990-2019. Front Public Health. 2022 Nov 29;10:952161. doi: 10.3389/fpubh.2022.952161. PMID: 36523572; PMCID: PMC9745318.
- Poddar KM, Chakraborty A, Banerjee S. Neurodegeneration: Diagnosis, Prevention, and Therapy. Oxidoreductase 2021.https://www.researchgate.net/publication/349117125_Neurodegeneration_Diagnosis_Prevention_and_Therapy
- Shusharina N, Yukhnenko D, Botman S, Sapunov V, Savinov V, Kamyshov G. Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression. Diagnostics. 2023; 13(3). /pmc/articles/PMC9914271/
- Xiao N, Le QT. Neurotrophic Factors and Their Potential Applications in Tissue Regeneration. Arch Immunol Ther Exp (Warsz). 2016 Apr;64(2):89-99. doi: 10.1007/s00005-015-0376-4. Epub 2015 Nov 26. PMID: 26611762; PMCID: PMC4805470.
- Pramanik S, Sulistio YA, Heese K. Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy. Mol Neurobiol. 2017 Nov;54(9):7401-7459. doi: 10.1007/s12035-016-0214-7. Epub 2016 Nov 5. PMID: 27815842.
- Amyotrophic Lateral Sclerosis (ALS). National Institute of Neurological Disorders and Stroke. 2024. https://www.ninds.nih.gov/health-information/disorders/amyotrophic-lateral-sclerosis-als
- Keon M, Musrie B, Dinger M, Brennan SE, Santos J, Saksena NK. Destination Amyotrophic Lateral Sclerosis. Front Neurol. 2021 Mar 29;12:596006. doi: 10.3389/fneur.2021.596006. PMID: 33854469; PMCID: PMC8039771.
- Amyotrophic lateral sclerosis (ALS). Symptoms and causes - Mayo Clinic. 2024. https://www.mayoclinic.org/diseases-conditions/amyotrophic-lateral-sclerosis/symptoms-causes/syc-20354022
- Roos RAC. Huntington’s disease: A clinical review. Orphanet J Rare Dis. 2010; 5(1):1-8. https://ojrd.biomedcentral.com/articles/10.1186/1750-1172-5-40
- Huntington Disease Article 2024. https://www.statpearls.com/ArticleLibrary/viewarticle/23053
- Rojas NG, Cesarini ME, Peker G, Andres G, Prat D, Luis Etcheverry J. Review of Huntington’s Disease: From Basics to Advances in Diagnosis and Treatment. J Neurol Res. 2022; 12(3):93-113. https://www.neurores.org/index.php/neurores/article/view/721/701
- Deture MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Molecular Neurodegeneration 2019; 14:1; 1-18. https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-019-0333-5
- Abubakar MB, Sanusi KO, Ugusman A, Mohamed W, Kamal H, Ibrahim NH, Khoo CS, Kumar J. Alzheimer's Disease: An Update and Insights Into Pathophysiology. Front Aging Neurosci. 2022 Mar 30;14:742408. doi: 10.3389/fnagi.2022.742408. PMID: 35431894; PMCID: PMC9006951.
- Breijyeh Z, Karaman R. Comprehensive Review on Alzheimer's Disease: Causes and Treatment. Molecules. 2020 Dec 8;25(24):5789. doi: 10.3390/molecules25245789. PMID: 33302541; PMCID: PMC7764106.
- Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2018 Jun 18;6(6):CD001190. doi: 10.1002/14651858.CD001190.pub3. PMID: 29923184; PMCID: PMC6513124.
- Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003154. doi: 10.1002/14651858.CD003154.pub4. Update in: Cochrane Database Syst Rev. 2006;(2):CD003154. PMID: 16034889.
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014 Apr 23-30;311(16):1670-83. doi: 10.1001/jama.2014.3654. PMID: 24756517.
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2002;(2):CD001447. doi: 10.1002/14651858.CD001447. Update in: Cochrane Database Syst Rev. 2007;(1):CD001447. PMID: 12076411.
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu LM, Jacoby R; DART-AD investigators. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009 Feb;8(2):151-7. doi: 10.1016/S1474-4422(08)70295-3. Epub 2009 Jan 8. PMID: 19138567.
- Slade SC, Underwood M, McGinley JL, Morris ME. Exercise and Progressive Supranuclear Palsy: the need for explicit exercise reporting. BMC Neurol. 2019 Nov 29;19(1):305. doi: 10.1186/s12883-019-1539-4. PMID: 31783740; PMCID: PMC6884751.
- Tomlinson CL, Herd CP, Clarke CE, Meek C, Patel S, Stowe R, Deane KH, Shah L, Sackley CM, Wheatley K, Ives N. Physiotherapy for Parkinson's disease: a comparison of techniques. Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD002815. doi: 10.1002/14651858.CD002815.pub2. PMID: 24936965; PMCID: PMC7120367.
- Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2013 Jun 5;2013(6):CD003260. doi: 10.1002/14651858.CD003260.pub2. PMID: 23740535; PMCID: PMC7144738.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, Woods B, Beck C, Auer S, Lai C, Spector A, Fazio S, Bond J, Kivipelto M, Brodaty H, Rojo JM, Collins H, Teri L, Mittelman M, Orrell M, Feldman HH, Muñiz R. Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161-78. doi: 10.1159/000316119. Epub 2010 Sep 10. PMID: 20838046.
- Sakthiswary R, Raymond AA. Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen Res. 2012 Aug 15;7(23):1822-31. doi: 10.3969/j.issn.1673-5374.2012.23.009. PMID: 25624807; PMCID: PMC4302533.
- Sivandzade F, Cucullo L. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. Int J Mol Sci. 2021 Feb 22;22(4):2153. doi: 10.3390/ijms22042153. PMID: 33671500; PMCID: PMC7926761.
- Brainstorm Cell Therapeutics Inc. Treating neurodegenerative diseases with stem cell therapy. 2024. https://www.nature.com/articles/d43747-020-00415-7
- Varma SK, Hyder MK, Som S, Dhanabal S. Stem cell therapy: in treatment of neurodegenerative diseases. J Stem Cell Res Ther (Edmond). 2018; 4(1). https://medcraveonline.com/JSRT/JSRT-04-00105.php
- Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res Ther. 2010; 1(5):1-7. https://stemcellres.biomedcentral.com/articles/10.1186/scrt37
- Issa SS, Shaimardanova AA, Valiullin VV, Rizvanov AA, Solovyeva VV. Mesenchymal Stem Cell-Based Therapy for Lysosomal Storage Diseases and Other Neurodegenerative Disorders. Front Pharmacol. 2022 Mar 2;13:859516. doi: 10.3389/fphar.2022.859516. PMID: 35308211; PMCID: PMC8924473.
- Marsh SE, Blurton-Jones M. Neural stem cell therapy for neurodegenerative disorders: The role of neurotrophic support. Neurochem Int. 2017 Jun;106:94-100. doi: 10.1016/j.neuint.2017.02.006. Epub 2017 Feb 20. PMID: 28219641; PMCID: PMC5446923.
- Ravisankar P, Dhanavardhan K, Prathyusha K, Rajan KV. Stem cell therapy role in neurodegenerative disorders. Archives of Mental Health. 2018; 19(1):3.
- Vissers C, Ming GL, Song H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv Drug Deliv Rev. 2019 Aug;148:239-251. doi: 10.1016/j.addr.2019.02.007. Epub 2019 Feb 21. PMID: 30797953; PMCID: PMC6703981.
- Yan SS, Campos de Souza S, Xie ZD, Bao YX. Research progress in clinical trials of stem cell therapy for stroke and neurodegenerative diseases. Ibrain. 2023; 9(2):214-30. https://onlinelibrary.wiley.com/doi/full/10.1002/ibra.12095
- Fan Y, Goh ELK, Chan JKY. Neural Cells for Neurodegenerative Diseases in Clinical Trials. Stem Cells Transl Med. 2023 Aug 16;12(8):510-526. doi: 10.1093/stcltm/szad041. PMID: 37487111; PMCID: PMC10427968.
- Enzo Life Sciences. Stem Cell Therapy in Neurodegenerative Diseases. 2019. https://www.enzolifesciences.com/science-center/technotes/2019/june/stem-cell-therapy-in-neurodegenerative-diseases/
- Sugaya K, Vaidya M. Stem Cell Therapies for Neurodegenerative Diseases. Adv Exp Med Biol. 2018;1056:61-84. doi: 10.1007/978-3-319-74470-4_5. PMID: 29754175.
- Wei M, Yang Z, Li S, Le W. Nanotherapeutic and Stem Cell Therapeutic Strategies in Neurodegenerative Diseases: A Promising Therapeutic Approach. Int J Nanomedicine. 2023 Feb 3;18:611-626. doi: 10.2147/IJN.S395010. PMID: 36760756; PMCID: PMC9904216.
- Wang Q, Song LJ, Ding ZB, Chai Z, Yu JZ, Xiao BG, Ma CG. Advantages of Rho-associated kinases and their inhibitor fasudil for the treatment of neurodegenerative diseases. Neural Regen Res. 2022 Dec;17(12):2623-2631. doi: 10.4103/1673-5374.335827. PMID: 35662192; PMCID: PMC9165373.
- Pourjabbar B, Shams F, Moghadam M, Ahani-Nahayati M, Azari A, Sefat F, Keshel SH. Recent Emerging Trend in Stem Cell Therapy Risk Factors. Curr Stem Cell Res Ther. 2023;18(8):1076-1089. doi: 10.2174/1574888X18666221223104859. PMID: 36567298.
- Rahman MM, Islam MR, Islam MT, Harun-Or-rashid M, Islam M, Abdullah S. Stem Cell Transplantation Therapy and Neurological Disorders: Current Status and Future Perspectives. Biology (Basel). 2022; 11(1). /pmc/articles/PMC8772847/
- Sudhakar V, Richardson RM. Gene Therapy for Neurodegenerative Diseases. Neurotherapeutics. 2019 Jan;16(1):166-175. doi: 10.1007/s13311-018-00694-0. PMID: 30542906; PMCID: PMC6361055.
- Notarte KI, Catahay JA, Macasaet R, Liu J, Velasco JV, Peligro PJ, Vallo J, Goldrich N, Lahoti L, Zhou J, Henry BM. Infusion reactions to adeno-associated virus (AAV)-based gene therapy: Mechanisms, diagnostics, treatment and review of the literature. J Med Virol. 2023 Dec;95(12):e29305. doi: 10.1002/jmv.29305. PMID: 38116715.
- Zu H, Gao D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021 Jun 2;23(4):78. doi: 10.1208/s12248-021-00608-7. PMID: 34076797; PMCID: PMC8171234.
- Vaughan EE, DeGiulio JV, Dean DA. Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import. Curr Gene Ther. 2006 Dec;6(6):671-681. doi: 10.2174/156652306779010688. PMID: 17168698; PMCID: PMC4400175.
- Kanter J, Falcon C. Gene therapy for sickle cell disease: where we are now? Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):174-180. doi: 10.1182/hematology.2021000250. PMID: 34889358; PMCID: PMC8791177.
- Chulpanova DS, Shaimardanova AA, Ponomarev AS, Elsheikh S, Rizvanov AA, Solovyeva VV. Current Strategies for the Gene Therapy of Autosomal Recessive Congenital Ichthyosis and Other Types of Inherited Ichthyosis. Int J Mol Sci. 2022 Feb 24;23(5):2506. doi: 10.3390/ijms23052506. PMID: 35269649; PMCID: PMC8910354.
- Maule G, Arosio D, Cereseto A. Gene Therapy for Cystic Fibrosis: Progress and Challenges of Genome Editing. Int J Mol Sci. 2020 May 30;21(11):3903. doi: 10.3390/ijms21113903. PMID: 32486152; PMCID: PMC7313467.
- Mani S, Jindal D, Singh M. Gene Therapy, A Potential Therapeutic Tool for Neurological and Neuropsychiatric Disorders: Applications, Challenges and Future Perspective. Curr Gene Ther. 2023;23(1):20-40. doi: 10.2174/1566523222666220328142427. PMID: 35345999.
- Al-Saif AM. Gene therapy of hematological disorders: current challenges. Gene Ther. 2019 Aug;26(7-8):296-307. doi: 10.1038/s41434-019-0093-4. Epub 2019 Jul 12. PMID: 31300728.
- Kumar SR, Markusic DM, Biswas M, High KA, Herzog RW. Clinical development of gene therapy: results and lessons from recent successes. Mol Ther Methods Clin Dev. 2016 May 25;3:16034. doi: 10.1038/mtm.2016.34. PMID: 27257611; PMCID: PMC4879992.
- Matharu N, Ahituv N. Modulating gene regulation to treat genetic disorders. Nat Rev Drug Discov. 2020 Nov;19(11):757-775. doi: 10.1038/s41573-020-0083-7. Epub 2020 Oct 5. PMID: 33020616.
- Hibbitt O, Wade-Martins R. Physiologically-Regulated Expression Vectors for Gene Therapy. In: Targets in Gene Therapy. InTech. 2011.
- Picanço-Castro V, Pereira CG, Covas DT, Porto GS, Athanassiadou A, Figueiredo ML. Emerging patent landscape for non-viral vectors used for gene therapy. Nat Biotechnol. 2020 Feb;38(2):151-157. doi: 10.1038/s41587-019-0402-x. PMID: 32034383; PMCID: PMC7308177.
- Kariyawasam D, Alexander IE, Kurian M, Farrar MA. Great expectations: virus-mediated gene therapy in neurological disorders. J Neurol Neurosurg Psychiatry. 2020 Aug;91(8):849-860. doi: 10.1136/jnnp-2019-322327. Epub 2020 Jun 5. PMID: 32503884.
- Hitti FL, Yang AI, Gonzalez-Alegre P, Baltuch GH. Human gene therapy approaches for the treatment of Parkinson's disease: An overview of current and completed clinical trials. Parkinsonism Relat Disord. 2019 Sep;66:16-24. doi: 10.1016/j.parkreldis.2019.07.018. Epub 2019 Jul 13. PMID: 31324556.
- Fuller-Carter PI, Basiri H, Harvey AR, Carvalho LS. Focused Update on AAV-Based Gene Therapy Clinical Trials for Inherited Retinal Degeneration. BioDrugs. 2020 Dec;34(6):763-781. doi: 10.1007/s40259-020-00453-8. PMID: 33136237.
- Martier R, Konstantinova P. Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock. Front Neurosci. 2020 Sep 18;14:580179. doi: 10.3389/fnins.2020.580179. PMID: 33071748; PMCID: PMC7530328.
- Morgan D. Immunotherapy for Neurodegenerative Disorders. Oxford University Press. 2016; 1.
- Kwon S, Iba M, Kim C, Masliah E. Immunotherapies for Aging-Related Neurodegenerative Diseases-Emerging Perspectives and New Targets. Neurotherapeutics. 2020 Jul;17(3):935-954. doi: 10.1007/s13311-020-00853-2. PMID: 32347461; PMCID: PMC7222955.
- Nicoll J. https://meetings.bna.org.uk/bna2023/prog/programme-day/immunotherapy-in-neurodegenerative-diseases/. 2023. IMMUNOTHERAPY IN NEURODEGENERATIVE DISEASES.
- Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175-93. doi: 10.1146/annurev.neuro.31.060407.125529. PMID: 18352830; PMCID: PMC2561172.
- Mortada I, Farah R, Nabha S, Ojcius DM, Fares Y, Almawi WY, Sadier NS. Immunotherapies for Neurodegenerative Diseases. Front Neurol. 2021 Jun 7;12:654739. doi: 10.3389/fneur.2021.654739. PMID: 34163421; PMCID: PMC8215715.
- Chatterjee D, Kordower JH. Immunotherapy in Parkinson's disease: Current status and future directions. Neurobiol Dis. 2019 Dec;132:104587. doi: 10.1016/j.nbd.2019.104587. Epub 2019 Aug 25. PMID: 31454546.
- White AR, Hawke SH. Immunotherapy as a therapeutic treatment for neurodegenerative disorders. J Neurochem. 2003 Nov;87(4):801-8. doi: 10.1046/j.1471-4159.2003.02064.x. PMID: 14622111.
- Weissmiller AM, Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl Neurodegener. 2012; 1(1):1-9. https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/2047-9158-1-14
- Blesch A. Neurotrophic factors in neurodegeneration. Brain Pathol. 2006 Oct;16(4):295-303. doi: 10.1111/j.1750-3639.2006.00036.x. PMID: 17107599; PMCID: PMC8095767.
- Bhardwaj R, Deshmukh R. Neurotrophic factors and Parkinson’s disease. Clin Investig (Lond). 2017; 8(1):53-62. https://www.openaccessjournals.com/articles/neurotrophic-factors-and-parkinsons-disease-12412.html
- Ouaamari ElY, Bos VDJ, Willekens B, Cools N, Wens I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int J Mol Sci. 2023; 24(4). /pmc/articles/PMC9968045/
- Garcia de Yebenes J, Yebenes J, Mena MA. Neurotrophic factors in neurodegenerative disorders: model of Parkinson's disease. Neurotox Res. 2000;2(2-3):115-37. doi: 10.1007/BF03033789. PMID: 16787836.
- Boudreau RL, Davidson BL. RNAi therapy for neurodegenerative diseases. Curr Top Dev Biol. 2006;75:73-92. doi: 10.1016/S0070-2153(06)75003-7. PMID: 16984810.
- Gonzalez-Alegre P. Therapeutic RNA interference for neurodegenerative diseases: From promise to progress. Pharmacol Ther. 2007 Apr;114(1):34-55. doi: 10.1016/j.pharmthera.2007.01.003. Epub 2007 Jan 25. PMID: 17316816.
- Sah DW. Therapeutic potential of RNA interference for neurological disorders. Life Sci. 2006 Oct 4;79(19):1773-80. doi: 10.1016/j.lfs.2006.06.011. Epub 2006 Jun 15. PMID: 16815477.
- Orlacchio A, Bernardi G, Orlacchio A, Martino S. RNA interference as a tool for Alzheimer's disease therapy. Mini Rev Med Chem. 2007 Nov;7(11):1166-76. doi: 10.2174/138955707782331678. PMID: 18045220.