More Information
Submitted: March 03, 2025 | Approved: March 07, 2025 | Published: March 10, 2025
How to cite this article: Wei C, Yao W. Regulation of Fear Behavior by Microcircuits within the Mouse Amygdala. J Neurosci Neurol Disord. 2025; 9(1): 001-009. Available from: https://dx.doi.org/10.29328/journal.jnnd.1001105
DOI: 10.29328/journal.jnnd.1001105
Copyright License: © 2025 Wei C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Basolateral amygdala; Central amygdala; Glutamatergic neurons; GABAergic interneurons; Fear extinction; Microcircuits
Regulation of Fear Behavior by Microcircuits within the Mouse Amygdala
Cheng Wei* and Wen Yao*
Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence; Key Laboratory of Mental Health of the Ministry of Education; Guangdong Province Key Laboratory of Psychiatric Disorders; School of Basic Medical Sciences, Southern Medical University, Guangzhou, 510515, Guangdong, China
*Address for Correspondence: Wei Cheng, Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence; Key Laboratory of Mental Health of the Ministry of Education; Guangdong Province Key Laboratory of Psychiatric Disorders; School of Basic Medical Sciences, Southern Medical University, Guangzhou, 510515, Guangdong, China, Email: [email protected]
Wen Yao, Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence; Key Laboratory of Mental Health of the Ministry of Education; Guangdong Province Key Laboratory of Psychiatric Disorders; School of Basic Medical Sciences, Southern Medical University, Guangzhou, 510515, Guangdong, China, Email: [email protected]
Background: The amygdala is a core structure in the mammalian brain that processes emotion and memory. Its complex neuronal composition and intricate microcircuit mechanisms play key roles in behaviors such as fear, anxiety, and reward. The diversity of neuronal types and the dynamics of these microcircuits provide the neural foundation for the encoding and extinction of fear memories.
Aim: This is a retrospective review article summarizing recent research on the amygdala and fear behavior in mice, which is of significant importance in helping people to comprehensively understand and recognize that the amygdala is the core regulator of fear behavior.
Methodology: An extensive and systematic search of electronic databases (Medline, PubMed, Web of Science) using keywords related to the amygdala and the technologies involved in the study such as “mouse amygdala,” “basolateral amygdala (BLA),” “central amygdala (CeA),” “fear extinction,” “fear learning,” and “microcircuits.” Articles meeting the selection criteria were included as candidate references.
Results: By integrating recent findings from optogenetics, chemogenetics, and single-cell sequencing, this review reveals the interactions between glutamatergic projection neurons and GABAergic interneurons in the amygdala, the functional division between subnuclei, and the neural basis of cross-brain area coordination. Additionally, it discusses the technical challenges in amygdala research and future directions, providing theoretical support for understanding the pathophysiology of emotional disorders.
Conclusion: The amygdala is intimately linked to emotional health, playing a critical role in understanding the mechanisms underlying the development of psychiatric disorders such as anxiety, depression, addiction, and post-traumatic stress disorder (PTSD). Despite advances in methodologies such as in vivo calcium imaging, neural circuit tracing, and electrophysiological techniques, which are progressively uncovering the underlying mechanisms of amygdalar regulation of emotional behaviors, the intrinsic microcircuitry of the amygdala remains highly complex. Significant gaps persist, necessitating further exploration and refinement to elucidate unresolved aspects of its functional architecture and behavioral modulation.
The amygdala is a crucial hub for integrating emotion, memory, and autonomic responses. Its central role in emotional processing has been gradually established since the early 20th century with studies on Klüver-Bucy syndrome. In rodent models, the mouse amygdala, due to its highly conserved neural circuits and experimental manipulability, has become an ideal model for studying the neural mechanisms of emotion. Pavlovian fear conditioning is widely used to investigate neuronal associations and mechanisms involved in associative learning in the brain [1]. In the auditory fear conditioning of mice, neutral conditioned stimuli (CS) are paired with harmful unconditioned stimuli (US), allowing the mouse to acquire fear memories. After CS-US pairing, presenting only the CS can trigger conditioned fear responses [2,3], such as “freezing” and “avoidance.” Repeated presentation of CS alone leads to a gradual reduction in conditioned responses, a phenomenon known as fear extinction [4]. Fear extinction does not eliminate the initial fear memory but represents a new learning process, where the resulting extinction memory competes with the original fear memory and jointly determines the mouse’s fear level [5-7]. Impaired fear extinction is associated with the development and persistence of human anxiety disorders, notably post-traumatic stress disorder (PTSD), one of the most prevalent and distressing mental health conditions worldwide [5,8].
Fear extinction deficits, heightened anxiety, and avoidance of trauma-related stimuli are hallmark symptoms of PTSD, with fear extinction dysfunction being a characteristic symptom [9]. Studying the mechanisms of fear memory formation, especially extinction, is crucial for understanding the underlying principles of these mental disorders and identifying potential therapeutic targets. Previous studies have found that the amygdala is one of the most critical brain regions involved in both fear formation and extinction [10,11]. With recent advancements in molecular tagging technologies, optogenetics, and calcium imaging, significant progress has been made in understanding the classification of amygdala neurons and the analysis of microcircuits. However, the complexity of cellular heterogeneity and dynamic regulation mechanisms in the amygdala still require further refinement. This article focuses on the neuronal diversity and microcircuits in the mouse amygdala, systematically reviewing the subnuclear divisions, neuronal subtype characteristics, and their functional roles in fear memory. It also explores the synergistic interactions between the amygdala and other brain regions, such as the prefrontal cortex, hippocampus, and hypothalamus. Combining the latest experimental evidence, this review aims to provide a theoretical foundation for understanding the mechanisms of amygdala-related emotional disorders and developing intervention strategies.
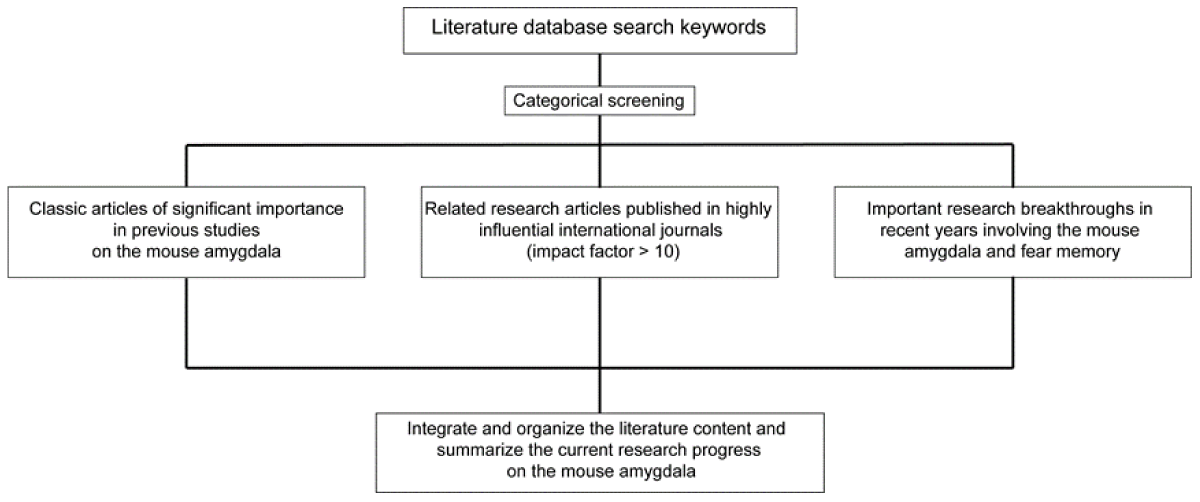
The references cited in this article were primarily sourced from the PubMed database using key search terms including “mouse amygdala,” “basolateral amygdala (BLA),” “central amygdala (CeA),” “fear extinction,” “fear learning,” and “microcircuits.” The inclusion criteria for selected references were defined as follows: first, seminal articles that established fundamental concepts in mouse amygdala research; second, studies published in high-impact international journals (impact factor >10); third, recent breakthroughs elucidating critical mechanisms of amygdala-mediated fear memory processes. Articles meeting any of these criteria were included as candidate references (Figure 1). After a comprehensive evaluation of all candidate literature, the authors objectively synthesized the scientific perspectives presented in these studies. This work declares no conflicts of interest with any cited authors or institutions, and the interpretation of referenced content was conducted with strict adherence to academic neutrality.
Figure 1: Literature collection and retrieval process.
Anatomical structure and functional division of the amygdala
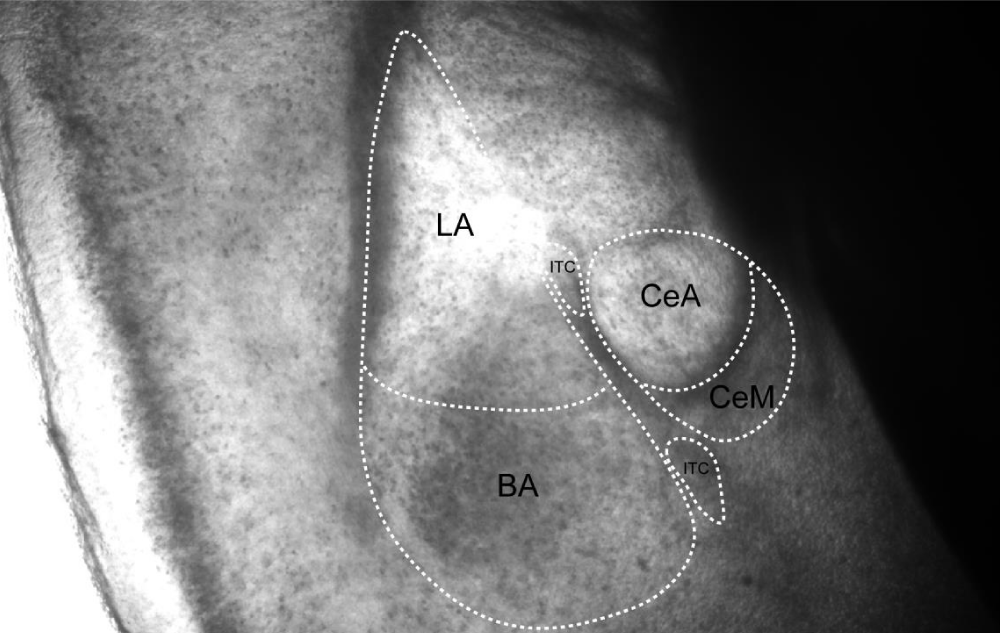
The mouse amygdala, located in the medial temporal lobe, consists of multiple subnuclei, including the basolateral amygdala (BLA) and the central amygdala (CeA). The BLA is further divided into the lateral amygdala (LA) and the basal amygdala (BA), while the CeA comprises the central core (CeC), lateral central nucleus (CeL), and medial central nucleus (CeM) (Figure 2). Additionally, several intercalated cell clusters (ITCs) surround the amygdala, serving as inhibitory nodes that mediate information transfer. The amygdala is one of the key brain regions for acquiring, expressing, and extinguishing conditioned fear behavior [12]. The BLA is considered a cortical-like structure and is the primary site where sensory information enters the amygdala. Neuronal activity in the BLA regulates the formation and retrieval of fear memories [13-15]. The CeA mainly consists of GABAergic inhibitory neurons and calibrates fear response levels. It receives projections from the BLA and is regarded as the output station of the amygdala complex [16]. ITCs, located around the amygdala, are inhibitory neuron clusters that mediate communication between the BLA and CeA, regulating the fear output level in the CeA [17].
Figure 2: Schematic diagram of the coronal section of the mouse amygdala, illustrating its fundamental subnuclear organization. The murine amygdala comprises three principal subnuclei: the basolateral amygdala (BLA), the central amygdala (CEA), and the intercalated interneuron clusters (ITC). The BLA is located laterally, the CEA occupies the medial region, and the ITC resides within the interposed lamina between the BLA and CEA.
Cell types and microcircuits in the Basolateral Amygdala (BLA)
The BLA consists of approximately 80% glutamatergic neurons and 20% GABAergic interneurons, playing a central role in processing, storing, and retrieving associative fear memories [18]. The BLA is histologically divided into the lateral (LA) and basal (BA) regions, which contain different types of principal neurons and have distinct neural circuit connectivity patterns. However, the interneuron populations in LA and BA are considered functionally homogeneous [19]. LA serves as the main input site for sensory information, receiving inputs from sensory cortices and the thalamus. It is crucial for forming CS-US associations. Interestingly, there is no direct connection between LA and CeM; information from LA is transmitted to CeM via BA [12,20]. The BLA contains two main types of neurons: glutamatergic pyramidal neurons and GABAergic interneurons. Glutamatergic pyramidal neurons exhibit typical cortical pyramidal morphology, while GABAergic interneurons have spiny or smooth dendrites, and their axonal branches are confined to the BLA [21]. The glutamatergic neurons in the BLA are involved in the storage of fear memories and respond to both positive and negative valence stimuli. Most BLA pyramidal neurons belong to two distinct genetic, functional, and anatomical populations [22]. The Rspo2+ and Ppp1r1b+ neurons in the BLA not only regulate fear levels within the amygdala but also participate in fear regulation in other brain regions outside the amygdala. One of the most widely studied regions in this regard is the medial prefrontal cortex (mPFC), which is another key brain area for fear learning and extinction behavior. Increasing evidence suggests that two subregions of the mPFC bidirectionally regulate conditioned fear responses, with the prelimbic cortex (PL) facilitating fear expression and the infralimbic cortex (IL) promoting fear extinction. The antagonistic effects of the PL and IL cortices on fear extinction are believed to be mediated through their interactions with the amygdala, as the PL and IL cortices receive distinct inputs from the Rspo2+ and Ppp1r1b+ neurons and project to different subregions of the amygdala, producing corresponding effects [23, 24].
While glutamatergic excitatory neurons in the BLA are crucial for memory formation and retrieval, increasing evidence suggests that their activity is highly coordinated through interactions with local GABAergic interneurons [25]. The interneurons in the BLA tightly regulate the excitability of glutamatergic neurons and play a key role in associative learning. Typical interneurons exhibit high-frequency firing, generate rapid, non-adaptive action potentials during prolonged depolarization, have narrower half-widths of action potentials, and larger afterhyperpolarization characteristics. The most extensively studied inhibitory interneurons in emotional learning are those expressing parvalbumin (PV) or somatostatin (SST). These two cell types constitute the majority of GABAergic interneurons and exhibit physiological and anatomical complementarity. PV produces high-frequency, non-adaptive action potentials, suppresses excitatory postsynaptic currents (EPSCs), and primarily forms synaptic contacts on the cell bodies, proximal axons, and dendrites of glutamatergic neurons. In contrast, SST preferentially targets the distal dendrites of excitatory neurons, enhancing EPSCs. Thus, the memory-related activity and plasticity of glutamatergic neurons in the BLA are modulated by PV and SST firing. Consistent with this view, further research has found that during fear learning,
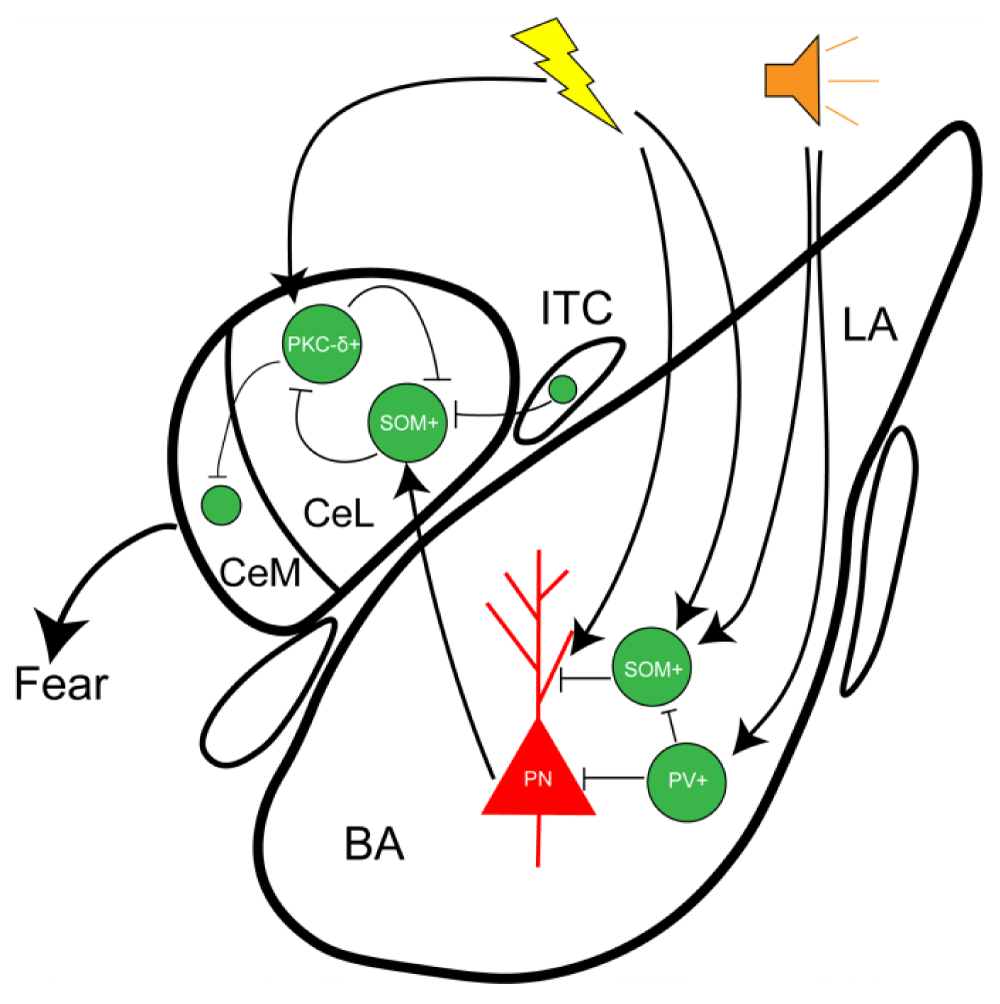
PV and SST in multiple brain regions show different responses to the conditioned stimulus (CS) and unconditioned stimulus (US). In the BLA, auditory CS stimulation increases PV firing activity while inhibiting SST firing activity [26]. Specifically, the processing of the auditory CS is controlled by a microcircuit in which PV and SST neurons regulate BLA input by inhibiting the dendrites of excitatory neurons during CS presentation. PV interneurons inhibit SST interneurons to indirectly disinhibit the dendrites of the principal neurons in the basolateral amygdala, thereby enhancing the auditory response and facilitating CS-US association. However, during the processing of the US, both PV and SST interneurons are inhibited, leading to disinhibition of the entire excitatory neuron, which strongly increases their activity, promoting postsynaptic harmful stimulus responses and fear learning. These results suggest that associative learning is dynamically regulated by the precise interactions of different interneuron subtypes within local microcircuits, with stimulus-specific activation of different cell types from various sources [27-29] (Figure 3).
Figure 3: The BLA receives and integrates sensory inputs from the thalamus and cortical regions, subsequently projecting to the lateral division of the central amygdala (CeL) to modulate fear expression levels. During fear learning, auditory stimuli enhance the activity of parvalbumin-positive (PV) neurons while suppressing somatostatin-expressing (SST) neurons. Both PV and SST neurons regulate fear output by inhibiting dendritic excitatory neurotransmission in the BLA during conditioned stimulus (CS) presentation, thereby controlling BLA-to-CeL projections and fine-tuning fear expression.
Cell types and microcircuits in the Central Amygdala (CeA)
Another important subnucleus of the amygdala, the central amygdala (CeA), is the output station of the fear circuit and has only recently become the focus of extensive research. Traditionally, the CeA was considered a homogeneous structure, serving as a passive relay station to send fear outputs to brain regions associated with fear effects, such as the lateral hypothalamus and periaqueductal gray. However, recent studies have shown that the structure and function of the CeA are not so simple. Compared to the BLA, which consists of 80% glutamatergic excitatory neurons and 20% GABAergic inhibitory neurons, it is commonly believed that the central amygdala (CeA) is almost entirely composed of GABAergic inhibitory neurons, with CeM output neurons using GABA for neurotransmission rather than glutamate. Consistent with this, few neurons in the CeA express glutamate transporters 1 and 2 [30]. However, similar to the BLA, the CeA is crucial for appetite and defense behaviors, containing several anatomically, molecularly, and functionally defined neuronal subgroups. The CeA consists of three subnuclei: the central capsule (CEC), the lateral (CEL), and the medial (CeM). Recent research has shown that these subnuclei are not only anatomically distinct but also functionally heterogeneous in their contributions to fear output, interacting to calibrate the level of fear responses [16,31]. These subnuclei play different roles in classical fear conditioning, with CEL being crucial for fear learning, CeM primarily involved in fear output, and CEC possibly providing an important Pavlovian association site, integrating direct harmful sensory signals and multimodal sensory information [32].In many studies of CEL, whole-cell patch-clamp electrophysiology, combined with molecular genetics and cell-based neuronal reconstruction, has distinguished two antagonistic neuronal populations within the CEL: neurons expressing protein kinase Cδ (PKCδ) are called CELoff, and those expressing somatostatin (SST) are called CELon. This has greatly enhanced our understanding of the cellular and functional heterogeneity mechanisms of CEL [33-35].
The electrophysiological and morphological characteristics of these two CeA neuronal subpopulations are significantly different. Compared to CELoff neurons, CELon neurons are more excitable, denser, and have more complex dendritic branching [36-38]. These findings support the view that genetically distinct CeA neurons have different functions and characteristics. The genetic anatomy of the functional connectivity between these two cell populations shows that CELon neurons can be activated by conditioned auditory stimuli and inhibit CELoff neurons. CELoff neurons primarily project and inhibit brainstem projection neurons in CeM, and the inhibition of CELoff neurons by CELon neurons can disinhibit CeM output neurons, thus producing a fear response [39]. This suggests that the level of conditioned fear response expression may be related to the plasticity changes in the activity of CELon and CELoff neurons. CELoff cells inhibit CeM output neurons, whereas CELon can disinhibit CeM output neurons by inhibiting CELoff, thereby increasing fear output [40,41]. Consistent with this, studies have found that pharmacogenetic silencing of PKCδ-expressing CELoff neurons enhances conditioned fear responses [40]. Therefore, the plasticity changes in the activity of these two types of neurons may be a critical step in fear extinction, and their activity is essential for the formation of extinction memory. In summary, the activity of CeA neuronal circuits is strictly regulated by local microcircuit interactions. CELoff and CELon neurons are closely connected in the circuit, and when certain stimuli or environments excite one population, the other is inhibited. This reciprocal inhibition represents a “winner-takes-all” model and is the circuit mechanism for switching between high and low fear states. During the conditioned reflex process, neuronal activity in the CEL is necessary for fear acquisition, and CEL may serve as an inhibitory interface, controlling CeM output by integrating sensory cortical and subcortical inputs.
The main output of the amygdala for fear responses is the central medial nucleus (CeM) [31]. Many studies have shown that projections from CeM in the amygdala to the periaqueductal gray control the expression of fear behavior [42]. The BLA can affect the CeM directly through glutamatergic projections or indirectly via a two-synapse pathway involving ITC or CEl neurons. Therefore, when the thalamus and sensory cortex transmit CS information into the BLA, the activity of the BLA increases the response of ITC or CEl neurons, regulating CeM output levels, which explains the reduction in fear expression after extinction [37,43]. The LA is the main target for thalamic and sensory cortical transmission of CS information. Although the LA is a key site for the plasticity of conditioned fear, it does not directly project to the CeM but instead indirectly influences the CeM via the BA. This suggests that the CeM receives glutamatergic projections from the BA, and its activity is influenced by the BA. In line with this, damage to the BLA after fear acquisition can eliminate conditioned fear responses [44, 45]. Furthermore, the activity of inhibitory long-range projection neurons in the CeM is necessary for the expression of conditioned fear responses, and their projections to the periaqueductal gray induce fear behaviors in mice, typically “freezing” and “escape” [46].
The central capsule (CEC) of the central amygdala receives various sensory information from the BLA and direct harmful information from the lateral parabrachial nucleus. Thus, the CEC serves as an information convergence point for the BLA and lateral parabrachial nucleus, potentially providing an important Pavlovian association site, integrating direct harmful sensory signals and various sensory information [32].
Intercalated cell clusters nediating BLA-CeA communication
In addition to the two major subnuclei of the amygdala, BLA and CeA, there are clusters of closely packed inhibitory interneurons surrounding the amygdala complex, known as the intercalated cell clusters (ITC), composed of densely arranged GABAergic interneurons surrounding the basolateral amygdala [17,47]. These inhibitory neuronal clusters mediate the communication between the BLA and CeA, providing feedforward inhibition to neurons in the CeA, and are believed to play a key role in fear extinction. Among these, the medial ITC cluster, which lies at the boundary between the BLA and CeA, has received widespread attention. It receives inputs from the BLA and transmits activity from the BLA and the prefrontal cortex, capable of inhibiting fear behavior by suppressing the output of the CeA. Sensory information from the thalamus and cortical areas can be directly transmitted to the interneurons in the ITC, which then send inhibitory projections to the BLA, effectively controlling the output of glutamatergic neurons in the BLA [48]. Therefore, the ITC not only provides feedforward inhibition to the glutamatergic principal neurons in the BLA, regulating their output activity but also sends projections to the CeA and transmits feedforward inhibition when receiving thalamic and cortical inputs, thereby inhibiting the activity of CeM output neurons and reducing fear responses.
The ITC is critical for the emotional processing of related amygdala functions, such as fear, anxiety, and reward. Dysfunction of the ITC has been shown to impair fear extinction, fear generalization, and social behavior. The ITC exerts control over the CeM through direct inhibition and feedforward inhibition, regulating the interaction between the BLA and the mPFC [48, 49]. Another insight into the ITC comes from a recent study showing that the activity balance between ITC clusters regulates the transition between high and low fear states [17]. Combined with the expression of genes associated with psychiatric disorders in the ITC, this makes the ITC a potential pharmacological target for fear- and anxiety-related diseases [31].
In summary, the ITC plays a key role in modulating the amygdala circuit and processing the information flow between the BLA and CeA. On the input side, the ITC receives primary sensory and higher-order associative information, driving its feedforward inhibition gate in the BLA. On the output side, the BLA drives the medial ITC cluster, leading to inhibition of CeA targets, providing a direct pathway to inhibit fear responses and thus providing feedforward inhibition to the CeM [50]. Extinction leads to an increase in the expression of immediate early genes in ITC cells, and damage to ITC after extinction leads to spontaneous recovery of conditioned fear responses. In addition, the ITC is also regulated by neuromodulators representing internal states, transmitting these signals to the CeA, BLA, and PFC, and then influencing downstream processes [9].
Microcircuit mechanisms of fear memory acquisition and extinction
The acquisition and extinction of fear memory are closely related to the neural circuits within the amygdala [7,51-53].
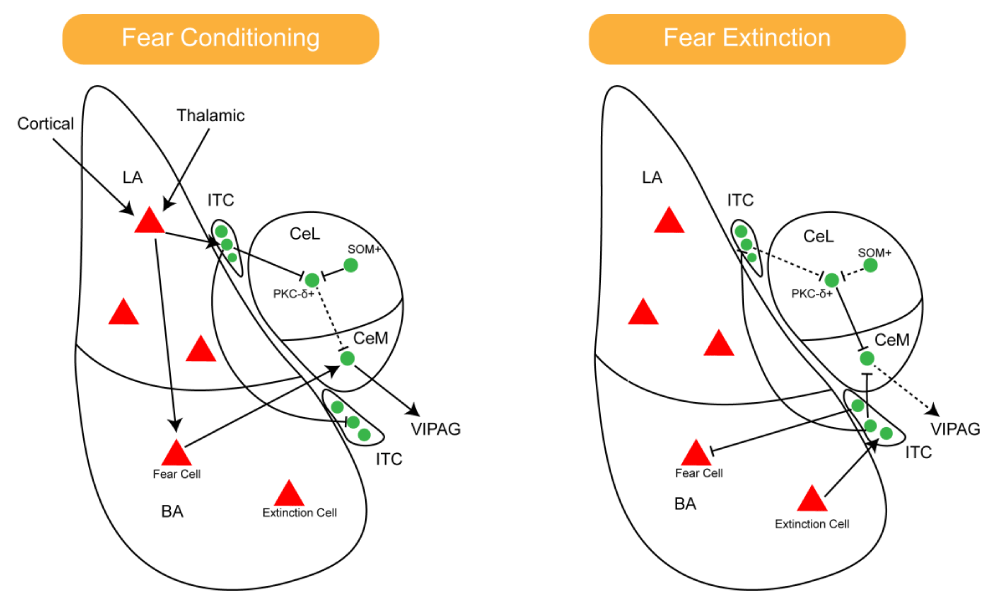
In the classical amygdala circuit for fear acquisition, the sensory cortex and thalamic inputs converge and are integrated into the BLA [54,55]. Subsequently, the BLA can directly excite the CeM, a subnucleus in the CeA responsible for fear output, which then sends downstream fear responses [56]. Additionally, the LA can project to the CEL, a subnucleus in the CeA involved in fear learning and regulation of fear levels, and through disinhibition of the CeA, increases fear output. In this process, the ITC mediates information transmission between the BLA and CeA [50] (Figure 4).
Figure 4: Dynamic activation patterns of amygdalar circuits during fear acquisition and extinction (solid lines denote activated and upregulated pathways, while dashed lines indicate inhibited pathways). Left: During fear acquisition, sensory inputs from the thalamus and cortex converge at the lateral amygdala (LA) for integration. Fear-encoding neurons in the basolateral amygdala (BA) directly excite the medial division of the central amygdala (CEM), which mediates downstream fear responses. Right: During fear extinction, extinction-specific neurons in the BLA exhibit heightened activity, projecting to both the CEM and ventral ITC neurons. The ITC, in turn, suppresses fear output from the CEM. Across both processes, the ITC serves as a critical relay for information transfer between the BLA and CeA.
During the acquisition of extinction, the activity of fear-suppressing projection neurons in the BLA increases. The BLA can influence the CeM and ITC neurons through direct glutamatergic projections, with the ITC inhibiting the CeM [7]. Therefore, when the BLA inputs the CS, activation of ITC or CEL neurons increases, which may explain the reduced fear expression after extinction. This is also controlled by multiple inputs, including those from the medial prefrontal cortex, that regulate ITC activity and reduce CeA output to suppress the fear response [4,57] (Figure 4).
Challenges and future directions in amygdala research
The amygdala in mice encodes fear information dynamically and produces behavioral outputs through molecularly defined neuronal types and multi-level microcircuits. The valence sorting in the BLA, hierarchical inhibition in the CeA, and gating function of the ITC together form the neural basis of the “fear-extinction” bistable system. Current research still has limitations, with technical constraints leading to an incomplete analysis of neuronal heterogeneity [26]. Existing molecular markers have not fully covered all interneuron subtypes, and there are some unexplained overlaps and cross-reactivities in the markers between different neuronal types. The advancement of single-cell sequencing technology in the future is expected to reveal more hidden cell populations. In studies investigating the mouse amygdala using in vivo calcium imaging and optogenetic techniques, several technical limitations persist. The temporal resolution of in vivo calcium imaging is constrained by the kinetic properties of calcium indicators, which may fail to capture millisecond-scale action potential dynamics [58,59]. Furthermore, spatial resolution is compromised in deep brain regions such as the amygdala, particularly in freely moving mice, where motion artifacts and signal drift may compromise data quality. For instance, in studies of basolateral amygdala (BLA)-to-central amygdala (CeA) projections, calcium imaging can record population-level neuronal activity but struggles to resolve precise temporal coding of individual neurons during complex behaviors [60,61]. Optogenetics relies on viral tools driven by specific promoters or projection-specific labeling, yet the high heterogeneity of amygdalar neuronal subtypes may lead to nonspecific activation or inhibition due to variable viral transfection efficiency or cross-projection interference. For example, studies have demonstrated that anterior cingulate cortex (ACC)-to-periaqueductal gray (PAG) projections specifically regulate pain-related affective states, whereas ACC-to-BLA projections lack this functional specificity, highlighting how targeting inaccuracies may confound experimental conclusions [62]. Additionally, the transient nature of optogenetic activation mismatches the extended temporal windows of behavioral experiments, potentially failing to fully replicate naturalistic neural activity patterns [63,64].In some key research areas, there are still aspects that require improvement. In the amygdala-prefrontal cortex circuit, the IL and PL cortices differentially project to the Rspo2+ and Ppp1r1b+ neurons in the BLA [22,23], which respectively promote fear extinction and expression, but the exact mechanism of their coordination remains unclear. Moreover, in the amygdala-hippocampal context encoding circuit, how hippocampal place cells integrate contextual information into fear memory through projections to the BLA still needs further exploration.
Future research on the amygdala may have medical applications, such as constructing targeted intervention strategies based on the specific regulation of PKCδ+ or SST+ neurons to develop anxiolytic drugs or gene therapies. Emerging clinical applications derived from research on the amygdala in anxiety and post-traumatic stress disorder (PTSD) include the following directions: First, the development of drugs targeting amygdalar neuroplasticity. Studies reveal that upregulation of the *Dcn* gene in the amygdala following traumatic brain injury (TBI) exacerbates fear conditioning by disrupting glutamate-GABA balance through modulation of perineuronal nets (PNNs). Inhibiting *Dcn* or regulating PNNs (e.g., via chondroitinase inhibitors to stabilize PNNs) may serve as novel therapeutic strategies to alleviate pathological fear memories in PTSD patients by rebalancing inhibitory neurotransmission in the amygdala [65]. Second, memory reconsolidation intervention strategies. In rodent models, fear memory reactivation combined with ketamine-targeted suppression of engram cells in the basolateral amygdala (BLA) effectively erases traumatic memories. This implies that NMDA receptor antagonists (e.g., ketamine) administered during the “fear-activated recall phase” of psychotherapy may enhance extinction efficacy, providing a pharmacological adjunct to clinical “retrieval-extinction therapy” [66]. Third, neural circuit-specific modulation. The central amygdala (CeA)-to-ventral bed nucleus of the stria terminalis (vBNST) projection pathway critically drives post-sepsis anxiety. Transient inhibition of this circuit using antiseizure agents (e.g., levetiracetam) may prevent trauma-related anxiety. Future approaches could employ deep brain stimulation (DBS) or focused ultrasound to selectively modulate this pathway in high-risk populations (e.g., severe infection survivors) for anxiety prophylaxis [67].
The mouse amygdala achieves precise encoding and dynamic regulation of emotional information through highly specialized neuronal types and multi-level microcircuit architecture. As a sensory integration center, the synaptic plasticity of glutamatergic neurons in the BLA serves as the cellular foundation of fear memory, while the CeA coordinates behavioral output through an inhibitory network of GABAergic neurons. With deepening understanding, it has become increasingly clear that the CeA plays a critical role in fear behavior regulation. It is recognized that the CeA is not just a passive fear output station but actively processes fear-related information on its own. The CeA is a key site for fear learning plasticity and is involved in fear extinction, not solely relying on inputs from the BLA to generate fear responses. The CeA can even form CS-US associations [68, 69]. Current research has confirmed that the amygdala plays a major role in emotion and memory functions, acting as the emotional processing center in the mammalian brain. The classical projections from the BLA→ITC→CeA have been shown to be involved in fear extinction. However, the local microcircuits between the CeA and other nuclei, as well as its role in fear memory and extinction, remain unclear and warrant further investigation [70,71]. To better understand the function of the amygdala, it is crucial to explore whether there are other local microcircuits, which neuronal subtypes participate in them, and how these circuits contribute to the regulation of fear behavior in animals, including their role in fear learning, expression, recollection, and extinction.
The amygdala stands as a central hub for deciphering the neural underpinnings of emotional behaviors and neuropsychiatric disorders, with its intricate microcircuitry serving as a cornerstone for understanding fear memory dynamics, valence processing, and behavioral adaptation. Amidst this critical role, persistent challenges emerge, including the unresolved complexity of intra-amygdalar microcircuit interactions, limitations in real-time monitoring of neuronal activity during naturalistic behaviors, and ethical considerations in translational studies involving genetic or pharmacological manipulations. Investigations into amygdalar function are further complicated by the heterogeneous nature of its neuronal subtypes and the dynamic interplay between glutamatergic and GABAergic networks.
This review summarizes recent advances in research on microcircuitry-mediated regulation of fear-related behaviors, advancing mechanistic insights into amygdala circuits and providing potential translational avenues for transforming these discoveries into targeted therapies for anxiety, PTSD, and other emotion-related disorders.
- Ledoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155-184. Available from: https://doi.org/10.1146/annurev.neuro.23.1.155
- Krabbe S, Gründemann J, Lüthi A. Amygdala inhibitory circuits regulate associative fear conditioning. Biol Psychiatry. 2018;83(10):800-809. Available from: https://doi.org/10.1016/j.biopsych.2017.10.006
- Maren S. Out with the old and in with the new: Synaptic mechanisms of extinction in the amygdala. Brain Res. 2015;1621:231-238. Available from: https://psycnet.apa.org/record/2014-53947-001
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31(4):599-612. Available from: https://doi.org/10.1111/j.1460-9568.2010.07101.x
- Barad M, Gean P, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60(4):322-328. Available from: https://doi.org/10.1016/j.biopsych.2006.05.029
- Paré D, Amano T, Unal CT. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13(4):489-494. Available from: https://doi.org/10.1038/nn.2499
- Ehrlich I, Humeau Y, Grenier F, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62(6):757-771. Available from: https://doi.org/10.1016/j.neuron.2009.05.026
- Lucas EK, Clem RL. GABAergic interneurons: The orchestra or the conductor in fear learning and memory? Brain Res Bull. 2018;141:13-19. Available from: https://doi.org/10.1016/j.brainresbull.2017.11.016
- Duvarci S, Paré D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82(5):966-980. Available from: https://doi.org/10.1016/j.neuron.2014.04.042
- Namkung H, Thomas KL, Hall J, Sawa A. Parsing neural circuits of fear learning and extinction across basic and clinical neuroscience: Towards better translation. Neurosci Biobehav Rev. 2022;134:104502. Available from: https://doi.org/10.1016/j.neubiorev.2021.12.025
- Warlow SM, Berridge KC. Incentive motivation: ‘Wanting’ roles of central amygdala circuitry. Behav Brain Res. 2021;411:113376. Available from: https://doi.org/10.1016/j.bbr.2021.113376
- Hunt S, Sun Y, Kucukdereli H, Sah P. Intrinsic circuits in the lateral central amygdala. eNeuro. 2017;4(1):316-367. Available from: https://doi.org/10.1523/eneuro.0367-16.2017
- Vereczki VK, Muller K, Krizsan E, Máté Z, Fekete Z, Rovira-Esteban L, et al. Total number and ratio of GABAergic neuron types in the mouse lateral and basal amygdala. J Neurosci. 2021;41(21):4575-4595. Available from: https://doi.org/10.1523/jneurosci.2700-20.2021
- Yau JO, Chaichim C, Power JM, McNally GP. The roles of basolateral amygdala parvalbumin neurons in fear learning. J Neurosci. 2021;41(44):9223-9234. Available from: https://doi.org/10.1523/jneurosci.2461-20.2021
- Esber GR, Holland PC. The basolateral amygdala is necessary for negative prediction errors to enhance cue salience, but not to produce conditioned inhibition. Eur J Neurosci. 2014;40(9):3328-3337. Available from: https://doi.org/10.1111/ejn.12695
- Keifer OJ, Hurt RC, Ressler KJ, Marvar PJ. The physiology of fear: Reconceptualizing the role of the central amygdala in fear learning. Physiol (Bethesda). 2015;30(5):389-401. Available from: https://doi.org/10.1152/physiol.00058.2014
- Hagihara KM, Bukalo O, Zeller M, Aksoy-Aksel A, Karalis N, Limoges A, et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nature. 2021;594(7863):403-407. Available from: https://doi.org/10.1038/s41586-021-03593-1
- Hájos N. Interneuron types and their circuits in the basolateral amygdala. Front Neural Circuits. 2021;15:687257. Available from: https://doi.org/10.3389/fncir.2021.687257
- Polepalli JS, Gooch H, Sah P. Diversity of interneurons in the lateral and basal amygdala. NPJ Sci Learn. 2020;5:10. Available from: https://doi.org/10.1038/s41539-020-0071-z
- Liu J, Hu T, Zhang M, Xu CY, Yuan MY, Li RX. Differential efferent projections of GABAergic neurons in the basolateral and central nucleus of amygdala in mice. Neurosci Lett. 2021;745:135621. Available from: https://doi.org/10.1016/j.neulet.2020.135621
- Sun Y, Gooch H, Sah P. Fear conditioning and the basolateral amygdala [version 1; peer review: awaiting peer review]. F1000Research. 2020;9:53. Available from: https://doi.org/10.12688/f1000research.21201.1
- Servonnet A, Hernandez G, El Hage C, Rompré PP, Samaha AN. Optogenetic activation of the basolateral amygdala promotes both appetitive conditioning and the instrumental pursuit of reward cues. J Neurosci. 2020;40(8):1732-1743. Available from: https://doi.org/10.1523/jneurosci.2196-19.2020
- Zhang X, Kim J, Tonegawa S. Amygdala reward neurons form and store fear extinction memory. Neuron. 2020;105(6):1077-1093. Available from: https://doi.org/10.1016/j.neuron.2019.12.025
- Choi K, Park K, Lee S, Yi JH, Woo C, Kang SJ, et al. Auditory fear conditioning facilitates neurotransmitter release at lateral amygdala to basal amygdala synapses. Biochem Biophys Res Commun. 2021;584:39-45. Available from: https://doi.org/10.1016/j.bbrc.2021.11.014
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60(5):765-773. Available from: https://doi.org/10.1016/j.neuropharm.2010.11.006
- Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Müller C, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509(7501):453-458. Available from: https://doi.org/10.1038/nature13258
- McDonald AJ, Zaric V. Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience. 2015;294:82-100. Available from: https://doi.org/10.1016/j.neuroscience.2015.03.004
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31(9):1664-1670. Available from: https://doi.org/10.1111/j.1460-9568.2010.07223.x
- Morrison DJ, Rashid AJ, Yiu AP, Yan C, Frankland PW, Josselyn SA. Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiol Learn Mem. 2016;135:91-99. Available from: https://doi.org/10.1016/j.nlm.2016.07.007
- Ciocchi S, Herry C, Grenier F, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277-282. Available from: https://doi.org/10.1038/nature09559
- Duvarci S, Popa D, Paré D. Central amygdala activity during fear conditioning. J Neurosci. 2011;31(1):289-294. Available from: https://doi.org/10.1523/jneurosci.4985-10.2011
- Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain. 2013;6:11. Available from: https://doi.org/10.1186/1756-6606-6-11
- Yu K, Ahrens S, Zhang X, Schiff H, Ramakrishnan C, Fenno L, et al. The central amygdala controls learning in the lateral amygdala. Nat Neurosci. 2017;20(12):1680-1685. Available from: https://doi.org/10.1038/s41593-017-0009-9
- Jo YS, Namboodiri VMK, Stuber GD, Zweifel LS. Persistent activation of central amygdala CRF neurons helps drive the immediate fear extinction deficit. Nat Commun. 2020;11(1):422. Available from: https://doi.org/10.1038/s41467-020-14393-y
- Yang T, Yu K, Zhang X, et al. Plastic and stimulus-specific coding of salient events in the central amygdala. Nature. 2023;616(7957):510-519. Available from: https://doi.org/10.1038/s41586-023-05910-2
- Hartley ND, Gaulden AD, Báldi R, Winters ND, Salimando GJ, Rosas-Vidal LE, et al. Dynamic remodeling of a basolateral-to-central amygdala glutamatergic circuit across fear states. Nat Neurosci. 2019;22(12):2000-2012. Available from: https://doi.org/10.1038/s41593-019-0528-7
- Whittle N, Fadok J, Macpherson KP, Nguyen R, Botta P, Wolff SBE, et al. Central amygdala micro-circuits mediate fear extinction. Nat Commun. 2021;12(1):4156. Available from: https://doi.org/10.1038/s41467-021-24068-x
- Adke AP, Khan A, Ahn HS, Becker JJ, Wilson TD, Valdivia S, et al. Cell-Type Specificity of Neuronal Excitability and Morphology in the Central Amygdala. eNeuro. 2021;8(1). Available from: https://doi.org/10.1523/eneuro.0402-20.2020
- Ressler RL, Maren S. Synaptic encoding of fear memories in the amygdala. Curr Opin Neurobiol. 2019;54:54-59. Available from: https://doi.org/10.1016/j.conb.2018.08.012
- Ressler RL, Goode TD, Evemy C, Maren S. NMDA receptors in the CeA and BNST differentially regulate fear conditioning to predictable and unpredictable threats. Neurobiol Learn Mem. 2020;174:107281. Available from: https://doi.org/10.1016/j.nlm.2020.107281
- Giovanniello J, Yu K, Furlan A, Nachtrab GT, Sharma R, Chen X, Li B. A Central Amygdala-Globus Pallidus Circuit Conveys Unconditioned Stimulus-Related Information and Controls Fear Learning. J Neurosci. 2020;40(47):9043-9054. Available from: https://doi.org/10.1523/jneurosci.2090-20.2020
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519(7544):455-459. Available from: https://doi.org/10.1038/nature13978
- McCullough KM, Chatzinakos C, Hartmann J, Missig G, Neve RL, Fenster RJ, et al. Genome-wide translational profiling of amygdala Crh-expressing neurons reveals role for CREB in fear extinction learning. Nat Commun. 2020;11(1):5180. Available from: https://doi.org/10.1038/s41467-020-18985-6
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017;93(6):1464-1479. Available from: https://doi.org/10.1016/j.neuron.2017.02.034
- Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci. 2017;20(10):1384-1394. Available from: https://doi.org/10.1038/nn.4623
- Yu K, Garcia Da Silva P, Albeanu DF, Li B. Central Amygdala Somatostatin Neurons Gate Passive and Active Defensive Behaviors. J Neurosci. 2016;36(24):6488-6496. Available from: https://doi.org/10.1523/jneurosci.4419-15.2016
- Asede D, Bosch D, Lüthi A, Ferraguti F, Ehrlich I. Sensory Inputs to Intercalated Cells Provide Fear-Learning Modulated Inhibition to the Basolateral Amygdala. Neuron. 2015;86(2):541-554. Available from: https://doi.org/10.1016/j.neuron.2015.03.008
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642-645. Available from: https://doi.org/10.1038/nature07167
- Asede D, Doddapaneni D, Bolton MM. Amygdala Intercalated Cells: Gate Keepers and Conveyors of Internal State to the Circuits of Emotion. J Neurosci. 2022;42(49):9098-9109. Available from: https://doi.org/10.1523/jneurosci.1176-22.2022
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16(6):317-331. Available from: https://doi.org/10.1038/nrn3945
- Choi DI, Kim J, Lee H, Kim JI, Sung Y, Choi JE, et al. Synaptic correlates of associative fear memory in the lateral amygdala. Neuron. 2021;109(17):2717-2726. Available from: https://doi.org/10.1016/j.neuron.2021.07.003
- Bocchio M, Nabavi S, Capogna M. Synaptic Plasticity, Engrams, and Network Oscillations in Amygdala Circuits for Storage and Retrieval of Emotional Memories. Neuron. 2017;94(4):731-743. Available from: https://doi.org/10.1016/j.neuron.2017.03.022
- Kasugai Y, Vogel E, Hörtnagl H, Schönherr S, Paradiso E, Hauschild M, et al. Structural and Functional Remodeling of Amygdala GABAergic Synapses in Associative Fear Learning. Neuron. 2019;104(4):781-794. Available from: https://doi.org/10.1016/j.neuron.2019.08.013
- Izquierdo I, Furini CRG, Myskiw JC. Fear Memory. Physiol Rev. 2016;96(2):695-750. Available from: https://doi.org/10.1152/physrev.00018.2015
- Butler CW, Wilson YM, Mills SA, Gunnersen JM, Murphy M. Evidence that a defined population of neurons in lateral amygdala is directly involved in auditory fear learning and memory. Neurobiol Learn Mem. 2020;168:107139. Available from: https://doi.org/10.1016/j.nlm.2019.107139
- Perumal MB, Sah P. Inhibitory Circuits in the Basolateral Amygdala in Aversive Learning and Memory. Front Neural Circuits. 2021;15:633235. Available from: https://doi.org/10.3389/fncir.2021.633235
- Ogden KK, Khatri A, Traynelis SF, Heldt SA. Potentiation of GluN2C/D NMDA Receptor Subtypes in the Amygdala Facilitates the Retention of Fear and Extinction Learning in Mice. Neuropsychopharmacology (New York, N.Y.). 2014;39(3):625-637. Available from: https://doi.org/10.1038/npp.2013.241
- Courtin J, Bitterman Y, Müller S, Hinz J, Hinz J. A neuronal mechanism for motivational control of behavior. Science (New York, N.Y.). 2022;375(6576):eabg7277.378-389. Available from: https://doi.org/10.1126/science.abg7277
- Chen APF, Chen L, Shi KW, Cheng E, Ge S, Xiong Q. Nigrostriatal dopamine modulates the striatal-amygdala pathway in auditory fear conditioning. Nat Commun. 2023;14(1):7231. Available from: https://doi.org/10.1038/s41467-023-43066-9
- Botta P, Fushiki A, Vicente AM, Hammond LA, Mosberger AC. An Amygdala Circuit Mediates Experience-Dependent Momentary Arrests during Exploration. Cell. 2020;183(3):605-619.e22. Available from: https://doi.org/10.1016/j.cell.2020.09.023
- Choi J, Lee YB, So D, So D, Kim JY. Cortical representations of affective pain shape empathic fear in male mice. Nat Commun. 2025. Available from: https://www.nature.com/articles/s41467-025-57230-w
- Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS. Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron. 2018;97(3):670-683.e6. Available from: https://doi.org/10.1016/j.neuron.2018.01.016
- Dalmay T, Abs E, Poorthuis RB, Hartung J, Pu DL. A Critical Role for Neocortical Processing of Threat Memory. Neuron. 2019;104(6):1180-1194.e7. Available from: https://doi.org/10.1016/j.neuron.2019.09.025
- Shi Y, Wu X, Zhou J, Cui W, Wang J. Single-Nucleus RNA Sequencing Reveals that Decorin Expression in the Amygdala Regulates Perineuronal Nets Expression and Fear Conditioning Response after Traumatic Brain Injury. Adv Sci (Weinh). 2022;9(7):e2104112. Available from: https://doi.org/10.1002/advs.202104112
- Li M, Yang X-K, Yang J, Li T-X, Cui C. Ketamine ameliorates post-traumatic social avoidance by erasing the traumatic memory encoded in VTA-innervated BLA engram cells. Neuron. 2024. Available from: https://doi.org/10.1016/j.neuron.2024.06.026
- Bourhy L, Bourhy L, Bourhy L, Mazeraud A, Mazeraud A. Silencing of amygdala circuits during sepsis prevents the development of anxiety-related behaviours. Brain. 2022. Available from: https://doi.org/10.1093/brain/awab475
- Avolio E, Alo R, Mele M, Carelli A, Canonaco A, Bucarelli L, et al. Amygdalar excitatory/inhibitory circuits interacting with orexinergic neurons influence differentially feeding behaviors in hamsters. Behav Brain Res. 2012;234(1):91-99. Available from: https://doi.org/10.1016/j.bbr.2012.06.013
- Kim J, Pignatelli M, Xu S, et al. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016;19(12):1636-1646. Available from: https://doi.org/10.1038/nn.4414
- Singh S, Topolnik L. Inhibitory circuits in fear memory and fear-related disorders. Front Neural Circuits. 2023;17:1122314. Available from: https://doi.org/10.3389/fncir.2023.1122314
- Heinrichs SC, Leite-Morris KA, Guy MD, Goldberg LR, Young AJ, Kaplan GB. Dendritic structural plasticity in the basolateral amygdala after fear conditioning and its extinction in mice. Behav Brain Res. 2013;248:80-84. Available from: https://doi.org/10.1016/j.bbr.2013.03.048
- Kwon OB, Lee JH, Kim HJ, Lee S, Lee S, Jeong MJ, et al. Dopamine Regulation of Amygdala Inhibitory Circuits for Expression of Learned Fear. Neuron. 2015;88(2):378-389. Available from: https://doi.org/10.1016/j.neuron.2015.09.001