More Information
Submitted: June 03, 2025 | Approved: June 09, 2025 | Published: June 10, 2025
How to cite this article: Gomes RR. The Rarest Case of Acute Bulbar Palsy due to Internal Jugular Vein Thrombosis Secondary to Protein S Deficiency: Vernet Syndrome. J Neurosci Neurol Disord. 2025; 9(1): 052-055. Available from:
https://dx.doi.org/10.29328/journal.jnnd.1001110
DOI: 10.29328/journal.jnnd.1001110
Copyright License: © 2025 Gomes RR. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Dural venous sinus thrombosis; Vernet syndrome; Protein S; Dabigatran
The Rarest Case of Acute Bulbar Palsy due to Internal Jugular Vein Thrombosis Secondary to Protein S Deficiency: Vernet Syndrome
Richmond Ronald Gomes*
Associate Professor, Medicine, Ad-Din Women’s Medical College Hospital, Bangladesh
*Address for Correspondence: Dr. Richmond Ronald Gomes, Associate Professor, Medicine, Ad-Din Women’s Medical College Hospital, Bangladesh, Email: [email protected]
Dural Venous Sinus Thrombosis (DVST) is a rare although serious clinical entity that causes approximately 0.5% of all stroke cases. Head trauma with skull base fracture, aneurysm, CNS infection, thrombophilia, and vasculitis may be identified as a possible cause of DVST. Vernet’s Syndrome is characterized by a constellation of unilateral cranial nerve palsies involving the 9th, 10th, and 11th cranial nerves due to compression or narrowing of the jugular foramen. We herein present a case of 33 years old Bangladeshi worker from Malaysia who had history of severe Traumatic Brain Injury (TBI) following road traffic accident with multiple skull bone fracture and extradural hematoma 3 months back, presented with acute dysphagia, dysphonia, fever and cough for 6 days. Neurologic examination revealed deviation of uvula to the left side and features of consolidation over right upper chest. Magnetic Resonance Venography (MRV) revealed thrombosis involving right transverse sinus, sigmoid sinus extending up to right internal jugular vein. The diagnosis of vernet syndrome with aspiration pneumonia was made. Later thrombophilia screen showed protein S deficiency. He was treated with broad spectrum antibiotics and started anticoagulation with dabigatran. After 6 months of anticoagulation he recovered fully with no residual neurological deficit.
Anterolateral to the foramen magnum there are the two jugular foramina, on either side of the skull base, the glossopharyngeal (IX), vagus (X), and spinal accessory (XI) nerves and internal jugular vein (IJV) are the structures that travel through this foramen. A fibro-osseous bridge that joins the jugular process of the occipital bone with the jugular spine of the temporal bone divides the foramen into two sections. The inferior petrosal sinus, the tympanic branch of IX (Jacobson’s nerve), and the cranial nerve IX are located in the anteromedial compartment (pars nervosa). The IJV, jugular bulb, cranial nerves X and XI, the auricular branch of vagus (Arnold’s nerve), and the posterior meningeal branch of the ascending nerve are all located in the poster lateral component, also known as the pars venosa or vascularis.
Dural Venous Sinus Thrombosis (DVST) is a rare although serious clinicopathological entity that causes approximately 0.5% of all stroke cases [1]. Superior sagittal sinus as well as transverse sinus are more affected than other dural sinuses [1]. Vernet’s Syndrome is characterized by a constellation of unilateral cranial nerve palsies due the compression or narrowing of the jugular foramen involving the 9th, 10th, and 11th cranial nerves (nerves that travel within the jugular foramen). A diversity of lesions has been shown to be involve in the jugular foramen, such as tumors (glomus jugulare, vestibular schwannoma, metastatic cancer), vascular lesions (mycotic aneurysm of the extracranial internal carotid artery after acute otitis media, internal jugular vein thrombosis following head trauma, CNS infection or due to thrombophilia), infections (meningitis, malignant otitis externa, varicella zoster virus), and trauma, inflammation (sarcoidosis, giant cell arteritis, SLE) [2].
Here we describe the case of a 33-year-old man who reported the occlusion of the right transverse sinus, right sigmoid sinus, and right internal jugular vein as the consequence of the severe traumatic brain injury with multiple skull bone fracture with development of epidural hematoma with superadded protein S deficiency. The patient was treated conservatively through the administration of Low Molecular Weight Heparin (LMWH) later with dabigatran for six months. We discuss the physio pathological hypothesis, the clinic-radiological aspects as well as the management options reviewing the literature.
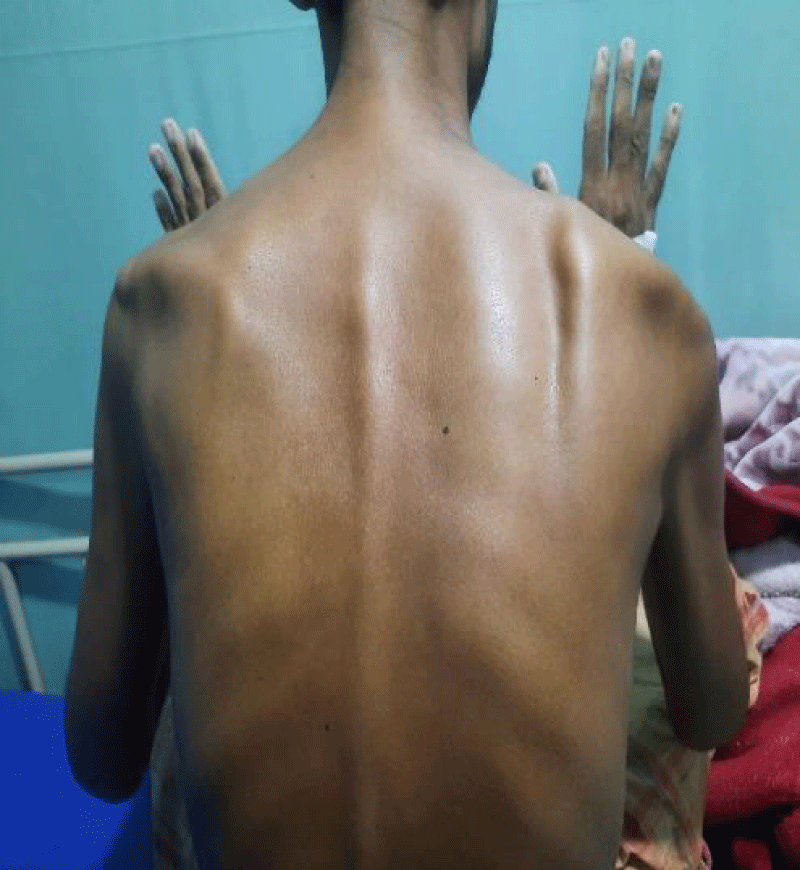
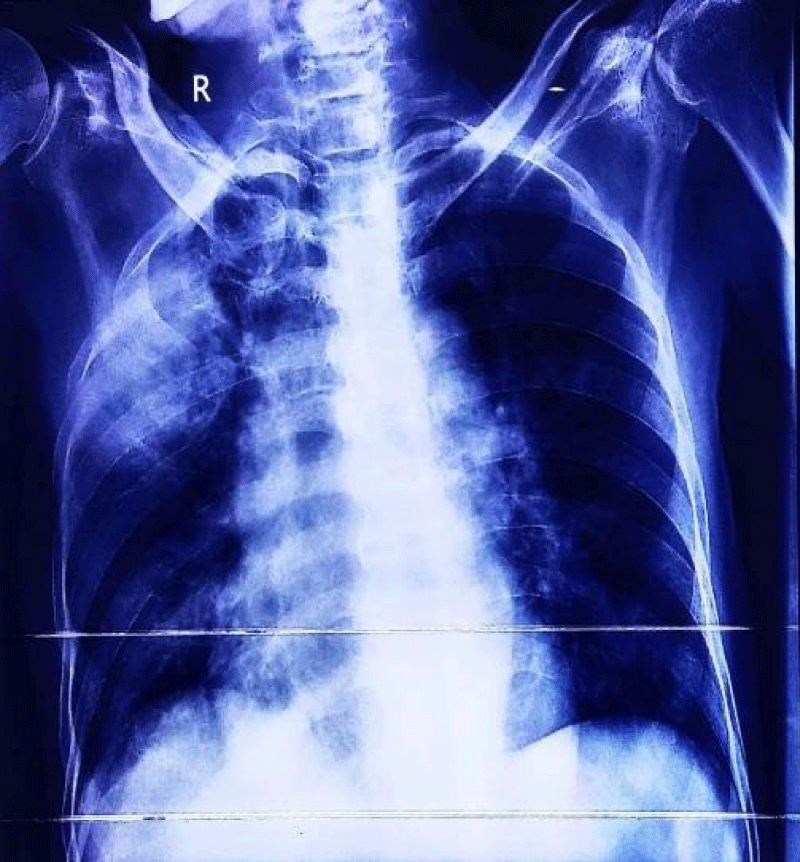
A 33-year-old industrial worker hailing from rural Bangladesh presented with sudden onset of inability to swallow both solid and liquids, voice change for 6 days, fever and worsening cough with expectoration of sputum for 3 days. Fever was high grade, intermittent. Highest recorded temperature was 103˚F. He denied any headache, altered sensorium, convulsion, shortness of breath, hemoptysis, chest pain, any limb weakness or any sphincteric or sensory disturbances. On query there was nasal regurgitation of food materials. On general examination he was febrile with pulse 102 beats/min, regular, BP 100/60 mm of Hg, respiratory rate 16 breaths/min. Chest examination revealed bronchial breathing with increased vocal resonance over right upper chest. Neurological examination revealed patient was conscious, oriented with right sided palatal palsy with absent gag reflex and hoarse voice, reduced muscle power over right trapezius and right sternocleidomastoid muscle with lateral winging of right scapula (Figure 1). Rest of other neurological examination revealed no abnormalities. On past history, he had road traffic accident three months back and hospitalized in intensive care unit at Malaysia for 1 month with the diagnosis of severe traumatic brain injury with left temporal and cerebellar extradural hematoma, with multiple facial and skull bone fracture and upper GI bleed from stress ulcer. He underwent bifrontal decompressive craniectomy. Initial investigation revealed, neutrophilic leukocytosis with total count 17,800/cmm with neutrophil 82%, ESR was 113 mm in 1st hour, CRP was 237 mg/dl. Liver function tests, renal function tests and MRI brain revealed no abnormalities. Chest X ray showed consolidation over right upper chest (Figure 2).
Figure 1: Showing lateral winging of right scapula.
Figure 2: Showing consolidation over right upper lung.
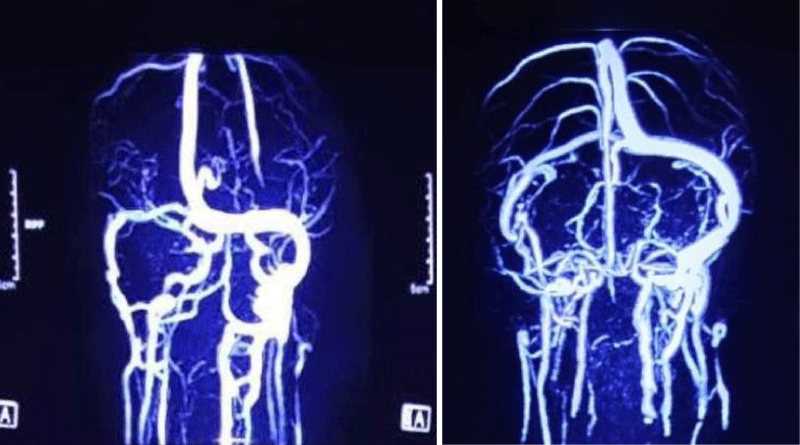
EMG showed increased insertional activities in the right sternocleidomastoid, abnormal spontaneous activities at rest, and reduced interferential patterns on maximal volition in the right trapezius muscles. However, there were no abnormal spontaneous activities at rest in the muscles of the tongue, face, cervical spine, and left upper extremity. He started treatment for aspiration pneumonia. Later, sputum culture revealed growth of Klebsiella pneumoniae sensitive to meropenem. Further workup revealed normal vasculitic screen (ANA, p-ANCA, c-ANCA) and normal CSF study. MRV of the brain revealed a thrombus in the right transverse sinus, the right sigmoid sinus extending up to the right internal jugular vein (Figures 2,3). D-dimer was raised 7. 71 mg/l (normal 0.5 mg/l). Thrombophilia screen was done. Protein C, homocysteine, lupus anti-coagulant, anti-cardiolipin antibody was negative, but protein S level came low (45%, normal 70% - 140%).
Figure 3: MRV of brain showing thrombus in right transverse sinus, right sigmoid sinus extending up to right internal jugular vein.
So final diagnosis of jugular foramen syndrome (vernet syndrome) was made secondary to protein S deficiency. He was started anticoagulation with low molecular weight heparin, enoxaparin 60 mg subcutaneous twice daily bridging with oral dabigatran 150 mg twice daily. With treatment, patient became afebrile, cough reduced. He was kept on nasogastric feeding. On 11th day he was discharged with oral dabigatran and NG tube in situ. CRP and complete blood count came normal. On follow up at 2 months, he was stable, can take food orally but hoarse voice was persisting. Further follow up at 6 months, there was no evidence of bulbar palsy. His voice became normal and there was no nasal regurgitation or chocking while taking food. Follow up MRV was normal.
The present case involves Vernet syndrome caused by dural venous sinus and internal jugular vein thrombosis secondary to protein S deficiency in which the damage of the glossopharyngeal and vagus nerves were confirmed by neurological examination and MRV and the damage of the spinal accessory nerve was confirmed by an electrodiagnostic study.
Jugular foramen syndrome generally refers to paralysis of one or more nerves of four specific cranial nerves. It also refers to neurological symptoms involving the glossopharyngeal nerve, vagus nerve, and spinal accessory nerve. By narrowing definition, jugular foramen syndrome is then known as Vernet syndrome [2]. The glossopharyngeal nerve, the vagus nerve and the spinal accessory nerve emerge from the posterolateral sulcus of the medulla oblongata, pass through the basal cistern and finally exit the cranium through the jugular foramen [2]. The causes of Vernet syndrome are primary tumors such as paraganglioma, meningioma, and schwannoma, metastatic tumors at the skull base, inflammation such as meningitis and malignant otitis externa, and sarcoidosis, Guillain-Barre syndrome, and trauma [3]. Symptoms of this syndrome are consequences of paresis of the above-mentioned cranial nerves (9, 10, 11). Common symptoms are hoarseness, nasal regurgitation of food, dysphagia, decrease in the parotid gland secretion, sternocleidomastoid and trapezius muscles paresis.
The initial imaging study in the evaluation of patients with possible DST is usually a brain CT scan. Magnetic resonance imaging, as well as MR angiography and venography, provide us with the most sensitive tools for detecting DVST. The combination of these imaging modalities constitutes the study of choice in the diagnosis of DVST.
Identification and treatment of the underlying causes should represent the first step in the treatment of dural sinus occlusion. In case of extrinsic compression such as depressed skull fractures as well as and steroids has shown a good prognosis showing restored muscle strength epidural or subdural hematoma surgical removal of the identified source of compression has been advocated by several authors [4,5]. Vernet syndrome caused by varicella zoster virus treated combined therapy with antiviral agents in shoulder joint abduction with improved dysphagia and hoarseness [6,7]. Thrombophilia should be treated with at least 6 months anticoagulation with either of warfarin, rivaroxaban, apixaban or dabigatran. The EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients conducted by Inhaul, et al. [8] in 2010 concludes that patients with cerebral sinus thrombosis without contraindications for anticoagulant should be treated either with body weight-adjusted subcutaneous LMWH or with dose adjusted intravenous heparin. In addition, the study concluded that the use of LMWH should be considered safe despite the presence of intracranial hemorrhage. LMWH pharmacologically doesn’t possess thrombolytic action; given this the main purpose of their administration is to prevent recurrent thrombosis and appositional thrombus growth. Data collected from different studies confirm that DST patients display a high spontaneous and intrinsic thrombolytic potential, with recanalization rates of 60% during the first 20 days [9].
The limitations of this case report are as follows. First, the MRI of the temporal bone in this patient was not high enough in resolution or thin enough in section imaging; thus, the edema or contrast enhancement in the affected left lower cranial nerves could not be confirmed through MRI. Second, an early electrodiagnostic study for vagus nerves regarding the vocal cord paralysis and a follow-up electrodiagnostic study for left spinal accessory neuropathy with axonopathy confirmed in the previous examination were not conducted.
Vernet syndrome is a rare although serious condition described in literature. Most reports show good outcome and recovery, but it might be related to a poor recovery and even lead to death. Internal jugular vein thrombosis should be kept in mine in patients presenting with acute dysphagia and dysphonia. Thrombophilia screen should be done in young patients. The administration of anticoagulant therapy still remains cornerstone of management which causes progressive reabsorption of the thrombus which could allow the recanalization of the dural sinus and internal jugular vein.
- Filippidis A, Kapsalaki E, Patramani G, Fountas KN. Cerebral venous sinus thrombosis: review of the demographics, pathophysiology, current diagnosis, and treatment. Neurosurg Focus. 2009;27:E3. Available from: https://doi.org/10.3171/2009.8.focus09167
- Robbins KT, Fenton RS. Jugular foramen syndrome. J Otolaryngol. 1980;9:505–516. Available from: https://pubmed.ncbi.nlm.nih.gov/7206037/
- Ha SW, Kim JK, Kang SJ, Kim MJ, Yoo BG, Kim KS, et al. A case of Vernet's syndrome caused by non-specific focal inflammation of the neck. J Korean Soc Clin Neurophysiol. 2007;9:81–84. Available from: https://www.e-acn.org/journal/view.php?number=300
- Bakar B, Tekkök IH. Venous sinus thrombosis after closed head injury: case report. Ulus Travma Acil Cerrahi Derg. 2010;16:98–102. Available from: https://pubmed.ncbi.nlm.nih.gov/20209407/
- Caplan JM, Khalpey Z, Gates J. Closed traumatic head injury: dural sinus and internal jugular vein thrombosis. Emerg Med J. 2008;25:777–778. Available from: https://doi.org/10.1136/emj.2008.061952
- Hayashi T, Murayama S, Sakurai M, Kanazawa I. Jugular foramen syndrome caused by varicella zoster virus infection in a patient with ipsilateral hypoplasia of the jugular foramen. J Neurol Sci. 2000;172:70–72. Available from: https://doi.org/10.1016/s0022-510x(99)00263-4
- Kawabe K, Sekine T, Murata K, Sato R, Aoyagi J, Kawase Y, et al. A case of Vernet syndrome with varicella zoster virus infection. J Neurol Sci. 2008;270:209–210. https://doi.org/10.1016/j.jns.2008.03.005
- Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, Masuhr F; European Federation of Neurological Societies. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–1235. Available from: https://doi.org/10.1111/j.1468-1331.2010.03011.x
- Beer-Furlan A, de Almeida CC, Noleto G, Paiva W, Ferreira AA, Teixeira MJ. Dural sinus and internal jugular vein thrombosis complicating a blunt head injury in a pediatric patient. Childs Nerv Syst. 2013;29:1231–1234. Available from: https://doi.org/10.1007/s00381-013-2184-7