More Information
Submitted: September 03, 2025 | Approved: October 08, 2025 | Published: October 09, 2025
How to cite this article: Ajao FO, Shittu EA, Kalejaiye NO, Iyedupe MO, Balogun DA, Okunlola O, et al. Sodium Propionate Ameliorates Neuronal Impairment, Oxidative Stress, and Inflammation in High-fat Diet Streptozotocin-Induced Diabetic Rats. J Neurosci Neurol Disord. 2025; 9(2): 056-066. Available from:
https://dx.doi.org/10.29328/journal.jnnd.1001111
DOI: 10.29328/journal.jnnd.1001111
Copyright License: © 2025 Ajao FO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Diabetes mellitus; Sodium propionate; Brain neurotransmitters; Oxidative stress; Neuro-inflammation
Sodium Propionate Ameliorates Neuronal Impairment, Oxidative Stress, and Inflammation in High-fat Diet Streptozotocin-Induced Diabetic Rats
Folasade Omobolanle Ajao*, Esther Adeola Shittu, Noheem Olaoluwa Kalejaiye, Marcus Olaoye Iyedupe, Damilola Ayodeji Balogun, Oluwatosin Okunlola and Abiodun Toheeb Afolabi
Physiology Department, Faculty of Basic Medical Science, College of Health Science, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria
*Address for Correspondence: Folasade Omobolanle Ajao, Physiology Department, Faculty of Basic Medical Science, College of Health Science, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria, Email: [email protected]
Background: Diabetes affects brain neurotransmitters, elicits neurological disorders. However, the treatment remains a global issue. This study investigated the neuroprotective effect of sodium propionate in high-fat diet/streptozotocin-induced diabetic rats.
Methods: Fifty adult Wistar rats (100 - 150 g) were included. A freshly prepared 35 mgkg-1b.wt-1 streptozotocin was injected intraperitoneally to induce diabetes after 6 weeks of HFD feeding. The animals were divided into 5 groups (n = 10). Group 1: control; Group 2: control + 200 mg kg-1 b.wt-1 sodium propionate; Group 3: diabetic; Group 4 diabetic + 200 mgkg-1b.wt-1 sodium propionate, Group 5: diabetic + 200 mgkg-1b.wt-1 metformin, respectively. Blood and brain tissue samples were collected after sacrificing the animals for biochemical assay.
Results: Brain epinephrine, norepinephrine, dopamine, serotonin, nitric oxide, acetylcholinesterase, triglyceride, total cholesterol, low-density lipoprotein-cholesterol, tumor necrosis factor-alpha, interleukin-6, and interleukin-10, malondialdehyde, caspase-3, insulin, fasting blood glucose, oral glucose tolerance and glycated hemoglobin were significantly (p < 0.05) higher in diabetic rats. Brain superoxide dismutase, catalase, reduced glutathione, B-cell lymphoma-2, and high-density lipoprotein-cholesterol were significantly (p < 0.05) reduced. Sodium propionate supplementation reduced the brain epinephrine, norepinephrine, dopamine, serotonin, nitric oxide, acetylcholinesterase, malondialdehyde, tumor necrosis factor-alpha, interleukin-6, interleukin-10, triglyceride, total cholesterol, low-density lipoprotein-cholesterol, caspase-3, insulin, fasting blood glucose, oral glucose tolerance and glycated hemoglobin. Brain superoxide dismutase, catalase, reduced glutathione, B-cell lymphoma-2, and HDL-cholesterol increased.
Conclusion: Sodium propionate modulates the brain neurotransmitters, improves antioxidants, and reduces neuronal oxidative stress and inflammation. Sodium propionate restored normal brain neurotransmission and could help to treat diabetes-associated neurological disorders.
Diabetes mellitus, a metabolic and non-communicable disease, is continually rising in prevalence globally. The International Diabetes Federation (IDF) forecasts the diabetes burden to reach 783 million by 2045 [1].
Diabetes mellitus is characterized by chronically elevated blood glucose levels resulting from impaired carbohydrate, lipid, and fat metabolism due to absolute or relative insulin secretion from beta-cells of the pancreas or insensitivity to insulin action (insulin resistance) at peripheral tissues or a combination of both [2]. Poorly treated, prolonged elevated blood glucose levels and insulin resistance are implicated as the etiology for the progression of diabetes micro-vascular and macro-vascular pathological complications [3].
In diabetes, chronic hyperglycemia disturbs the physiological balance between the oxidative stress and antioxidant system, which consequent in high free radical production and a decline in antioxidant levels [4]. Oxidative stress and antioxidant defence disequilibrium facilitate chronic inflammation that favours pro-inflammatory cytokine release, both exacerbating diabetes-related organ complications, including the brain, eyes, kidney, heart, and liver [5].
The brain is a crucial organ that is highly sensitive to insulin. Insulin plays a key role in regulating neurotransmitter activity, differentiation and development of neurons, neuroplasticity, cognition, learning, glucose homeostasis, and appetite regulation [6]. However, the brain’s hippocampus is extremely susceptible to altered insulin signalling linked to diabetes [7]. Chronically elevated blood glucose and insulin resistance can lead to the dysregulation of brain metabolism, oxidative stress, inflammation, and neurotransmitters, which can increase the progression of cognitive impairment and neurodegenerative disorder, known as diabetic neuropathy [8]. Neuropathy is a microvascular complication currently affecting 50% of the global diabetes patients [9,10], and this manifests in various ways, including loss of cognitive function, impaired sensation, neuropathic pain, and autonomic dysfunction [11]. Despite numerous available drugs, such as metformin, the first-line drug with remarkable anti-hyperglycemic properties to treat diabetes. This medication displayed inferiority in completely curing diabetes, and prolonged utilization is associated with the risk of developing cognitive abnormalities and neuronal disorders in diabetes [12]. To date, no specific drug has been selected to manage neurotransmitter disturbances in diabetes, and the focus has been on searching for novel drugs from natural sources [13].
Propionate, butyrate, and acetate are short-chain fatty acids (SCFAs) consumed via diet or produced through fermentation of non-digestible fibre by the gut microbiota [14]. These SCFAs account for 90% produced by the gut microbiota [15]. SCFAs have many pharmacological properties beneficial to human health, such as immune booster, cardio-protective, anti-cancer, anti-obesity, liver protection, anti-diabetes, and central nervous system protective effect [16]. Recently, research confirmed that through the connection of the parasympathetic nervous system, SCFAs have both direct effects on beta-cells of the pancreas and indirect effects on insulin secretion [17]. Also, the blood glucose-lowering effect of SCFAs has been reported in different in vivo experimental diabetic animal studies [18-21]. Among the SCFAs, sodium propionate has been well studied both in in vivo investigations and clinical reports for its beneficial effect on acute motor coordination and sensory axonal neuropathy, multiple sclerosis, degenerative disease, and various other autoimmune disorders, primarily through immunomodulatory mechanisms [22]. As an agonist for G-protein-coupled receptor 41 (GPR41), propionate targets the enteric nervous system and exerts neuronal protective effects in mice with serotonin-induced Parkinson’s disease [23]. Nonetheless, a lack of literature still exists on the effect of this short-chain fatty acid on abnormal brain neurotransmitters associated with neurological disorders in diabetes. This present study investigates the neuroprotective effect of sodium propionate in high-fat diet and streptozotocin-induced diabetic rats..
Chemicals, drugs, and reagents
Streptozotocin (STZ) (Sigma-Aldrich Co., Germany), ketamine, xylazine, phosphate buffer, metformin, glucose, and normal saline. All chemicals and reagents used are analytical grade.
Experimental animals
Fifty mature male Wistar rats weighing 100 - 150 g) were obtained from the Animal Research House of Physiology Department, Ladoke Akintola University of Technology (LAUTECH), Ogbomoso, Oyo State, Nigeria. The rats were confined in a plastic polypropylene cage and acclimated for a week with free access to feed and water ad libitum in a pathogen-free environmental condition of temperature (25 °C - 27 °C), humidity (45% - 50%), and (12h light/12h dark) cycles. All procedures and handling of animals in this experiment were performed based on the guidelines of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Faculty of Basic Medical Sciences Research Committee of LAUTECH, with approval number: ERCFBMSLAUTECH: 093/02/2025.
Diabetes induction
The animals were allocated into two groups before diabetes induction based on the diet received. Animals were fed on a normal diet, and animals were fed on a high-fat diet (carbohydrate: 30%, fat: 65% and protein: 5%). Each group was fed with the diet and water ad libitum for 6 weeks. After this period, animals were placed on overnight fasting (10 – 12 h). Diabetes was induced in high-fat diet animals by intraperitoneal injection of a repeated dose of freshly prepared STZ 35 mgkg-1body weight-1. Hypoglycemic death was prevented by giving the animals a 2% glucose solution after streptozotocin injection. The blood glucose of the animals was checked after 72 h of streptozotocin injection to authenticate diabetes induction through a drop of puncture-tail vein blood placed on a glucose strip, and reading was taken with a one-touch active glucometer (Accu-Chek). Animals with fasting blood glucose levels higher than 200 mg/dL were chosen as a diabetic rat model for the experiment.
Experimental design
The animals were randomly allocated into two distinct groups: Control and HFD/STZ-induced diabetic groups. Ten animals on a normal diet served as the control and were grouped into 2 groups (n = 10). Thirty animals on HFD/STZ-induced diabetes were divided into 3 groups (n = 10). The animals were treated with sodium propionate (NaP) and Metformin (met) via oral administration as follows:
Group 1: Normal control (NC)
Group 2: NC + 200 mg kg-1 b.wt-1 NaP
Group 3: Diabetes mellitus (DM)
Group 4: DM + 200 mg kg-1b.wt-1 NaP
Group 5: DM + 200 mg kg-1b.wt-1 Met
Drug administration lasted for 4 weeks. Daily food intake, water intake, and weekly body weight changes were monitored and recorded throughout the treatment period. Blood glucose levels were checked weekly throughout the entire drug administration phase.
Oral glucose tolerance test
The animals were subjected to 12 h overnight fasting the day before the 14th day of the treatment period. Early morning of the 14th day, the animals’ fasting blood glucose level was checked via pricked tail vein blood at 0 min (before 2% oral glucose administration) and subsequently at 30, 60, 90, and 120 min after glucose ingestion with a blood glucometer.
Collection of blood and brain tissue
The animals fasted overnight (12 h) on the day the last dose of the drug was administered. The rats were anaesthetized with 40 mg kg-1b.wt-1 ketamine and 20 mg kg-1b.wt-1 xylazine to be unconscious and sacrificed by dislocation of the cervical using a humane method. Fasting blood samples were collected from the apex beat of the rats’ hearts via a cardiac puncture with a sterile needle and syringe. The blood samples were immediately centrifuged at 3500 rpm for 10 minutes in a cold-centrifuge at (-4 oC). The clear serum obtained was used for biochemical estimation.
The animals’ skulls were dissected after blood collection. The brain was isolated, rinsed in cold normal saline to remove blood stains, and homogenized in cold phosphate-buffered saline. The homogenized tissue was centrifuged at 5000 rpm for 15 min at -4 oC. The supernatant retrieved was used to analyze the brain chemical neurotransmitters, oxidative stress, inflammation, and apoptosis.
Biochemical assays
The glucose-oxidase/peroxidase was employed to determine the fasting blood glucose (FBG) levels with a kit. Enzyme-linked immunosorbent assay (ELISA) technique was used to estimate insulin levels in the serum with a rat ELISA kit according to the protocols of the manufacturer. Serum glycated hemoglobin (HbA1c) was assessed with a rat HbA1c assay kit following the manufacturer’s instructions.
Insulin resistance (IR) was calculated using the homeostatic model assessment index (HOMA-IR). HOMA−IR = [FBG (mg/dL) × fasting insulin (μU/mL)]/405
Levels of total cholesterol (TC), triglycerides (TG), and high-density lipoprotein-cholesterol (HDL-C) in the rat brain were measured using the enzymatic colorimetric method. Friedewald, et al. [24] formula was utilized to calculate brain low-density lipoprotein-cholesterol (LDL-C) (mmol/L): TC - HDL - TG/2.2.
The neurotransmitters epinephrine, norepinephrine, dopamine, serotonin, and acetylcholinesterase (AchE) in the rat brain were estimated based on the sandwich ELISA method using a commercially available rat ELISA assay kit specific to each parameter according to the manufacturer’s protocol. The method adopted by Matar et al. [25] was used to determine the levels of malondialdehyde (MDA), nitric oxide (NO), catalase (CAT), superoxide dismutase (SOD), and reduced glutathione (GSH) in the rat brain with respective kits according to the instructions of the manufacturer. Brain inflammatory cytokines, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10) were determined using the ELISA method following the manufacturer’s instructions on each ELISA kit. Apoptotic (caspase-3) and anti-apoptotic (B-cell lymphoma-2) markers in the rat brain were measured with the ELISA method as per the manufacturer’s instructions.
Statistical analysis
Data were expressed as the mean ± SEM. The data were analyzed with one-way analysis of variance (ANOVA) using GraphPad Prism (version 10.3 software), followed by a Bonferroni post hoc test for group significance difference comparison. The level of statistical significance was set at p < 0.05.
Effects of sodium propionate on body weight, food intake, and water intake in HFD/STZ-Induced diabetic rats
The body weight of HFD/STZ-induced diabetic rats was significantly reduced (p < 0.05) compared with the controls. While water intake and food intake in the diabetic rats were significantly (p < 0.05) increased compared with the control rats. Sodium propionate administration elevated the body weight and reduced the food intake and water intake in comparison with the diabetic rats (Table 1).
| Table 1: Effect of sodium propionate on body weight, food intake, and water intake in HFD/STZ-induced diabetic rats. | |||
| Parameters Groups | Body weight (g) | Food intake/rat/day (g) | Water intake/rat/day (ml) |
| Control | 262.75 ± 5.20 | 29.80 ± 0.66 | 38.20 ± 1.28 |
| Control + 200 mg/kg.wt NaP | 254.75 ± 4.10 | 27.20 ± 0.86 | 37.60 ± 2.25 |
| DM | 179.00 ± 5.28β | 39.40 ± 0.87β | 58.50 ± 1.17β |
| DM + 200 mg/kgb.wt NaP | 224.75 ± 4.59α | 30.60 ± 0.87α | 38.20 ± 1.28α |
| DM + 200 mg/kgb.wt Met | 232.50 ± 4.73α | 31.80 ± 1.07α | 39.80 ± 1.56α |
| Values presented as mean ± SEM (n = 10). βsignificant at p < 0.05 vs. control; αsignificant at p < 0.05 vs. diabetic. | |||
Effects of sodium propionate on serum OGTT, FBG, insulin, HOMAR-IR, HbA1c, and AUC in HFD/STZ-induced diabetic rats
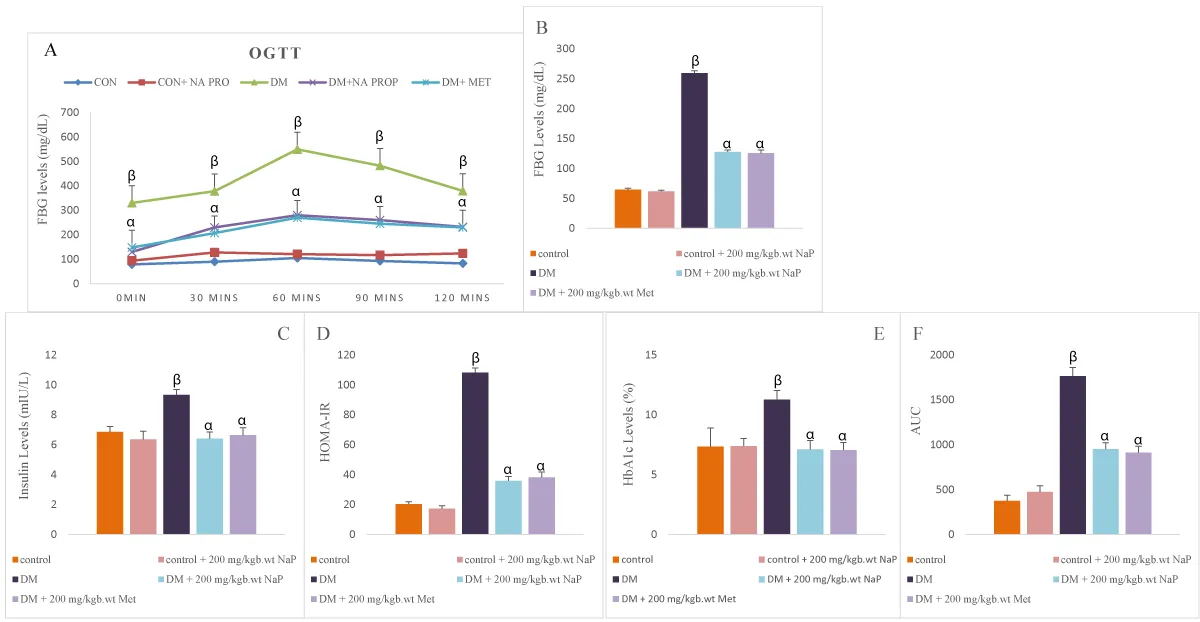
The OGTT levels in diabetic rats increased (p < 0.05) significantly compared with the controls. The OGTT levels reduced significantly in diabetic rats treated with sodium propionate in comparison with untreated diabetic rats (Figure 1A).
Serum FBG, insulin, and HbA1c significantly (p < 0.05) increased in the diabetic rat compared with controls. Treatment with sodium propionate significantly reduced the levels of serum FBG, insulin, and HbA1c compared with the diabetic rats (Figure 1B-E).
Area under the curve (AUC) in diabetic rats increased significantly (p < 0.05) compared with the controls. Administration of sodium propionate reduced the AUC significantly in diabetic rats in comparison with untreated diabetic rats (Figure 1F).
Figure 1: Effect of sodium propionate on serum (A) OGTT, (B) FBG, (C) insulin, (D) HOMA-IR, (E) HbA1c, (F) area under the curve in HFD/STZ-induced diabetic rats. Values presented as mean ± SEM (n=10). βsignificant at p < 0.05 vs. control; αsignificant at p < 0.05 vs. diabetic.
Effect of sodium propionate on brain neurotransmitters in HFD/STZ-induced diabetic rats
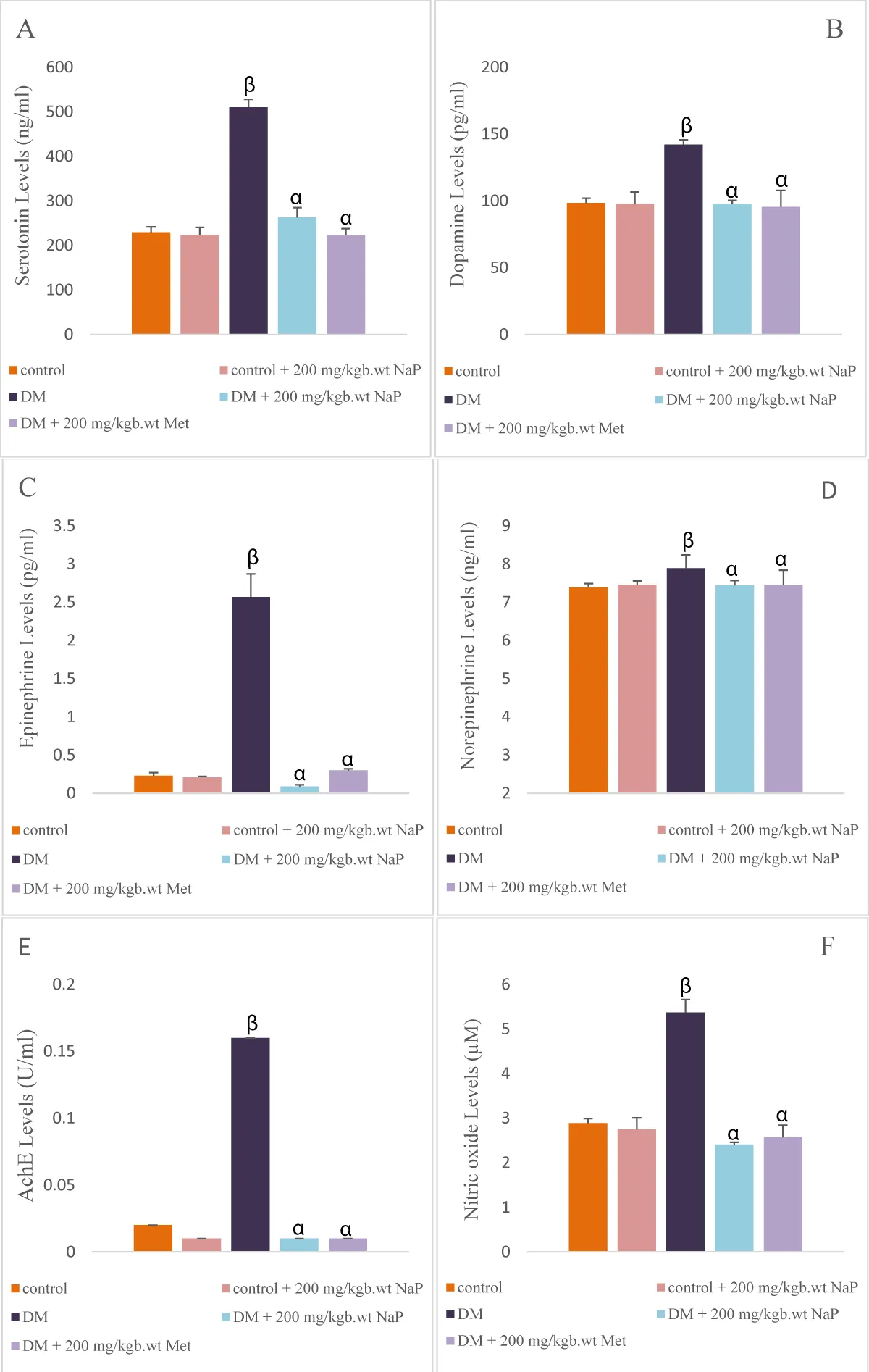
Serotonin, dopamine, epinephrine, norepinephrine, AchE, and nitric oxide levels in the brain of diabetic rats were significantly (p < 0.05) increased compared with the controls. Sodium propionate administration significantly reduced the levels of brain serotonin, dopamine, epinephrine, norepinephrine, AchE, and nitric oxide in comparison with the diabetic rats (Figure 2A-F).
Figure 2: Effect of sodium propionate on brain (A) serotonin, (B) dopamine, (C) epinephrine, (D) norepinephrine, (E) AchE, (F) nitric oxide in HFD/STZ-induced diabetic rats. Values presented as mean ± SEM (n = 10). βsignificant at p < 0.05 vs. control; αsignificant at p < 0.05 vs. diabetic.
Effect of sodium propionate on brain lipid profile in HFD/STZ-induced diabetic rats
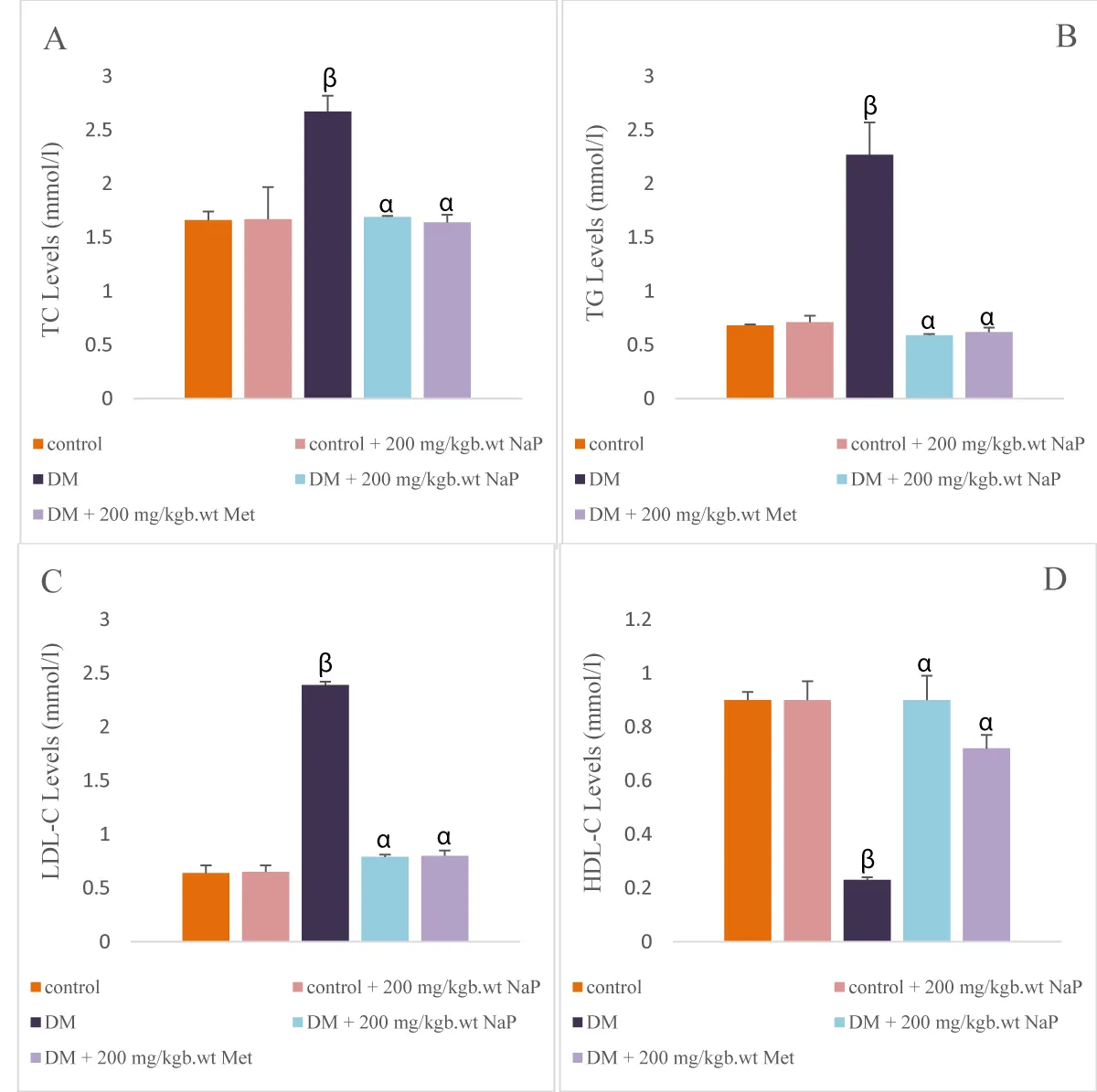
There was a significant (p < 0.05) increase in the brain TC, TG, and LDL-cholesterol levels in the diabetic rats in comparison with the controls. HDL-cholesterol levels reduced (p < 0.05) significantly in the diabetic compared with controls.
Administration of sodium propionate significantly reduced the levels of TC, TG, LDL-cholesterol and elevated the HDLL-cholesterol levels in comparison with the diabetic rats (Figure 3 A-D).
Figure 3: Effect of sodium propionate on brain (A) TC, (B) triglycerides, (C) LDL-C, (D) HDL-C in HFD/STZ-induced diabetic rats. Values presented as mean ± SEM (n = 10). βsignificant at p < 0.05 vs. control; αsignificant at p < 0.05 vs. diabetic.
Effects of sodium propionate on brain oxidative stress and antioxidants in HFD/STZ-induced diabetic rats
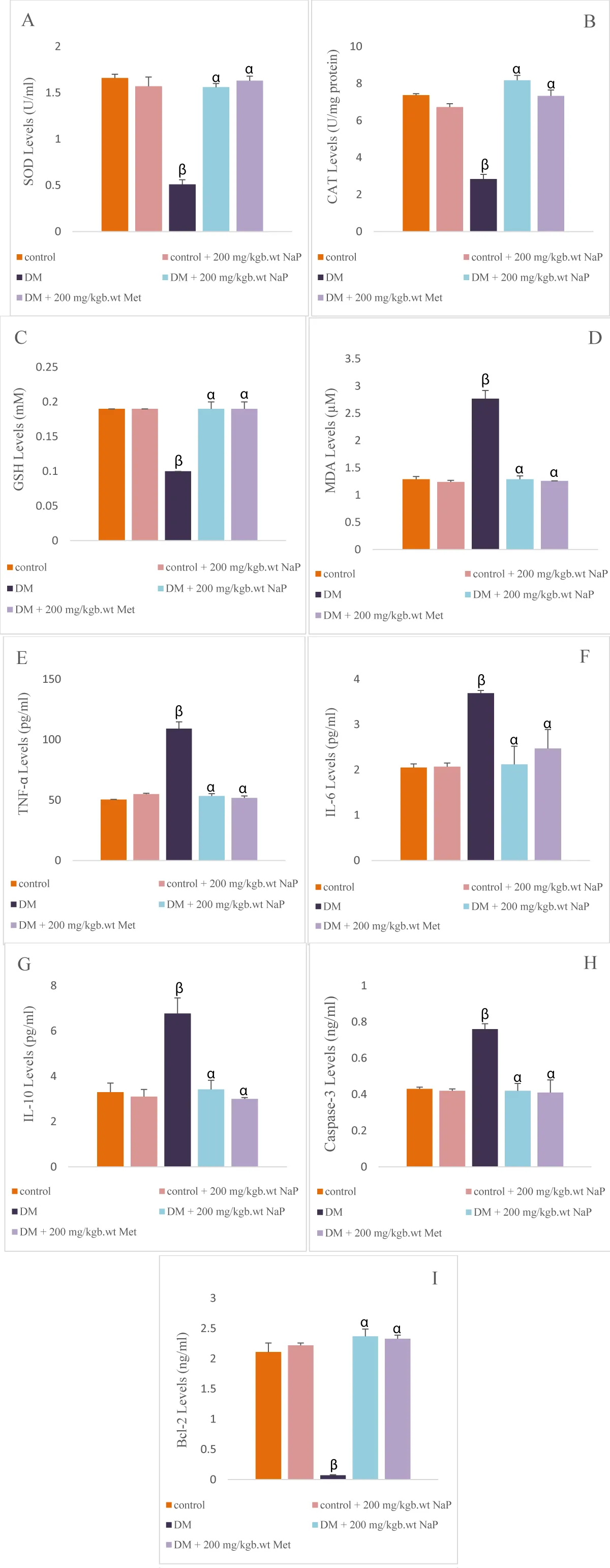
SOD, CAT, and GSH levels in the brain of diabetic rats were significantly (p < 0.05) reduced when compared with the controls. MDA levels in the diabetic rat brain significantly (p < 0.05) increased compared with the controls. Administration of sodium propionate increased the SOD, CAT, and GSH levels significantly in comparison with the diabetic rats. Also, sodium propionate administration significantly reduced the brain MDA levels in comparison with the diabetic rats (Figure 4A-D).
Figure 4: Effect of sodium propionate on brain (A) SOD, (B) CAT, (C) GSH, (D) MDA, (E)TNF-α, (F) IL-6, (G) IL-10, (H) caspase-3, (I) Bcl-2 in HFD/STZ-induced diabetic rats. Values presented as mean ± SEM (n = 10). βsignificant at p < 0.05 vs. control; αsignificant at p < 0.05 vs. diabetic.
Effect of sodium propionate on brain inflammatory markers in HFD/STZ-induced diabetic rats
The levels of brain TNF-α, IL-6, and IL-10 in diabetic rats were significantly (p < 0.05) elevated in comparison with the controls. Administration of sodium propionate decreased the levels of TNF-α, IL-6, and IL-10 significantly in comparison with the diabetic rats (Figure 4E-G).
Effect of sodium propionate on brain apoptotic and anti-apoptotic markers in HFD/STZ-induced diabetic rats
Caspase-3 levels increased (p < 0.05) significantly, and B-cell lymphoma-2 (Bcl-2) levels reduced (p < 0.05) significantly in the diabetic rat brain compared with the controls. Sodium propionate administration significantly reduced the brain caspase-3 levels and raised the Bcl-2 levels in comparison with the diabetic rats (Figure 4H, and I).
In diabetes conditions, alterations to glucose homeostasis have a deleterious effect on normal brain cell function and neuronal communication, consequently progressing to neurological complications in type-2 diabetes mellitus [26,27]. The exact mechanism underlying the pathogenesis of brain complications associated with diabetes is unclear, and studies have proposed that disruptions in neuronal insulin signaling affect the brain [25], leading to increased neurotransmitter activity, oxidative stress, and inflammatory responses [28]. Treating neurological disorders in diabetes with hypoglycemic drugs produced little satisfactory effect, and this requires discovering a promising drug for its management. Propionate of SCFAs has been recently studied for improving cognitive dysfunction and cerebral circulation in diabetic mice [19]. This study investigated the neuroprotective effects of sodium propionate in diabetic rats.
The symptoms clinically noticed in diabetes are hyperglycemia, polyphagia, polydipsia, polyuria, and body weight loss [29]. Insulin insufficiency or ineffectiveness in diabetes consequently leads to the breakdown of muscle structural protein, which causes body weight loss [30]. In line with the report, the diabetic rats in our study exhibited body weight loss, excess water, and food intake. However, the water and food intake were reduced, and the body weight was recovered with sodium propionate administration. These results suggest that the propionate had an anabolic activity by abrogating the degradation of structural protein and fat in muscle and increasing the insulin sensitivity for glucose uptake as energy.
Chronic hyperglycemia, high insulin levels, and impaired insulin sensitivity are known to be the etiology of type-2 diabetes [12]. Matar, et al. and Kumari, et al. reported lower insulin levels, increased blood glucose, and HOMA-IR in diabetic rats [25,31]. Contrary to those reports, another study observed hyperglycemia, a significant increase in insulin, HbA1c, and HOMA-IR in a diabetic encephalopathy animal model [13]. The current finding is consistent with the result, hyperglycemia accompanied by sustained hyperinsulinemia, elevated HOMA-IR, and HbA1c are manifested in the diabetic rats, suggesting the establishment of type-2-diabetes in our study. The relative or absolute insensitivity to insulin action by peripheral tissues for glucose uptake contributes to increased blood glucose levels and circulating insulin. Also, an increase in insulin levels observed serves as a compensatory mechanism in response to high blood glucose. Sodium propionate administered to the diabetic rats returned the blood glucose and insulin levels close to normal and improved the insulin sensitivity remarkably, as noticed by a reduction in HOMA-IR, HbA1c, and enhanced oral glucose tolerability, similar to Mandaliya et al.’s findings [18]. Sodium propionate may exert hypoglycemic properties through stimulating tissues and organs for efficient utilization of insulin for glucose uptake and reducing hepatic glycogenolysis and gluconeogenesis by activating 5’ adenosine monophosphate-activated protein kinase (AMPK) [32].
Neurotransmitters are natural body chemicals that send signals to various organs [33]. Hyperglycemia can disrupt the neurotransmitters in cholinergic, serotonergic, and dopaminergic systems, which significantly contribute to diabetes-related neurological disorders [34]. Acetylcholinesterase (AchE) is are cholinergic neurotransmitter enzymes that play a vital role in supporting cognition and memory in non-pathological conditions. Cognitive deficits and loss of memory are linked to the consequences of chronic hyperglycemia [33]. In the diabetic rat brain, increased AchE activity has been reported to cause cognitive dysfunction [35]. Similarly, this study observed a high level of the enzymes, which reveals cognitive function impairment in diabetic rats, which concurs with earlier reports linking insulin deficiency to cholinergic system disturbance and decline in mental function [36]. Dopamine is widely recognised as a crucial neurotransmitter in the brain, primarily for motor coordination and cognitive function through transmission in the dopaminergic circuit, and is degraded by monoamine oxidase [37,38]. The synthesis and release of dopamine may be altered, disturbing the reward center and possibly contributing to mood syndrome [39]. Low dopamine levels in the diabetic brain are linked with consequences of insulin malfunction in neuronal circuits [40]. Conversely, elevated brain dopamine level was observed in the diabetic rats of our study. Serotonin and norepinephrine are among the monoamine neurotransmitters significant for mood regulation, and alterations to the brain’s physiological levels of these neurotransmitters have been associated with different neuronal behavioural disorders [41]. In both human and animal diabetic experimental studies, impaired serotonin levels triggered depression, and norepinephrine deficiency disrupts the fight or flight behaviour [41]. The results of current findings in diabetic rats revealed elevated brain serotonin levels, while norepinephrine levels slightly increased, which aligns with a previous finding that reported an increase in diabetic brain neurotransmitters [42]. Sodium propionate supplementation reduced these neurotransmitters to a desirable level, suggesting that sodium propionate exhibits a positive restorative effect in the regulation of brain neurotransmitters to mitigate neuronal damage in hyperglycemic conditions.
Nitric oxide is an unsteady free radical in gaseous form that plays diverse roles under both normal and disease conditions [43]. Nitric oxide is an important signaling molecule that is involved in regulating blood vessel dilation, transmission across the synapse, and immune response. In type-2 diabetes, nitric oxide regulates vasodilation by promoting relaxation of blood vessels and improving blood flow [43]. Diabetes usually induces high nitric oxide production, which can damage the endothelial function and raise the oxidative stress, all contribute to vascular neuronal and cardiac complications of diabetes [44,45]. In corroborating the findings of Matar, et al. [25], the brain nitric oxide in diabetic rats of this study was high. However, sodium propionate administration lessens the nitric oxide level, showing inhibitory efficacy of the SCFAs to regulate nitric oxide release from the endothelium, thereby preventing neuronal complications.
Dyslipidemia includes high levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol is an autonomous factor for the development of cognitive loss in neurological disease [46,47]. In animal studies, elevated cholesterol can injure the blood-brain barrier and cause deterioration of the brain neuronal network [48]. Despite the lack of literature on the relationship between change in lipid profile and cognitive decline in diabetes. Interesting, this study observed brain dyslipidemia in diabetic rats, marked by a rise in triglycerides, total cholesterol, low-density lipoprotein-cholesterol levels and reduced levels of high-density lipoprotein-cholesterol. Sodium propionate administration to diabetic rats ameliorates the brain high-density lipoprotein-cholesterol, superseding the low-density lipoprotein-cholesterol and reduces the triglycerides and total cholesterol. This showed the anti-hyperlipidemic potent properties of sodium propionate in inhibiting the onset of neurological disorders associated with an altered brain lipid profile in diabetes independent of any other factors and could be attributed to inhibition of lipase enzymes and increased transport of cholesterol from tissue.
Diabetes progression is closely linked to the occurrence of oxidative stress, and the presence of hyperglycemia provokes reactive oxygen species (ROS) production. Overproduction of oxidative stress is implicated in compromising cognitive function [13]. It is well-documented that gluconeurotoxicity stems primarily from hyperglycemia-induced increased production of advanced glycation end-products (AGEs), enhanced activity of the polyol and hexosamine pathways, and activation of protein kinase C (PKC) isoforms, collectively contributing to neuronal oxidative damage [49]. Endogenous antioxidants are natural molecules that protect cells from oxidative damage [50]. Chronic hyperglycemia favours high oxidative stress that weakens the endogenous antioxidants, ultimately facilitating diabetic-related neuropathy complications [43]. In the current findings, an increase in malondialdehyde level, a marker of oxidative stress, and a decline in antioxidants (SOD, CAT, and GSH) were observed in the brain of diabetic rats, which agreed with the report of Singh, et al. [13]. Treatment with sodium propionate scavenged the free radicals of oxidative stress and ameliorated the level of antioxidants in the brain, connoting the anti-oxidative properties of the short-chain fatty acid in preventing cognitive loss related to neuronal oxidative damage.
The progression of neuro-inflammatory response that leads to neuronal injury involves an interconnection between hyperglycemia triggers oxidative stress, and induces neuronal dysfunction [51]. During inflammation, increased production of cytokines and reactive oxygen species (ROS) contributes to the development of cognitive impairment is facilitated by the activation of microglia by immunogenic molecules [13]. Recently, a study established that db/db mice with diabetes and obesity showed impairments in spatial-recognition memory with elevated levels of pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6, which signifies a potential link between inflammation and memory worsening [52]. In line with the previous report by Esmaeili, et al. [53], this study found a significant upsurge in the activity of pro-inflammatory cytokines TNF-α, IL-6, and IL-10 in the brain of diabetic rats. Nevertheless, supplementation of sodium propionate attenuates these pro-inflammatory cytokines in diabetic rat brain, demonstrating the anti-inflammatory potential of sodium propionate in abolishing brain neuro-inflammation in diabetes to improve the memory function, which might stem from its antioxidant efficacy.
Neuronal inflammation and oxidative stress produce free radicals in the brain that interact with the cell apoptosis mechanism physiological process [54]. Cell apoptosis occurs between the protein member caspase-3 and B-cell lymphoma-2 (Bcl-2) [55]. Glucotoxicity affects brain cell survival by favouring the overexpression of caspase-3 and down-regulating the expression of anti-apoptotic marker Bcl-2, which are key factors in the dopaminergic neuronal death in Parkinson’s disease and progression of neurodegenerative disease [56]. Present findings observed elevated brain caspase-3 and lower Bcl-2 in diabetic rats, similar to Nasiry et al findings [57]. Administration of sodium propionate lessens the caspase-3 and enhances the anti-apoptosis protein (Bcl-2),
suggesting its anti-apoptotic properties to prevent brain neuronal death. This efficacy may be mediated through the enhancement of brain antioxidants, which inhibit neuronal inflammation.
Sodium propionate exhibited glycemic control, modulated brain neurotransmitters, and alleviated hyperglycemia-related neuronal oxidative stress and inflammation. Sodium propionate could be exploited as an innovative therapy to enhance neuronal function and communication for preventing neuronal loss and progression of neurological disorders in diabetes.
However, a clinical trial is recommended to established these sodium propionate therapeutic efficacy in human and its non-toxicity to other organs.
Ethical considerations
The protocol was approved by the Faculty of Basic Medical Sciences Research Committee of LAUTECH, with approval number: ERCFBMSLAUTECH: 093/02/2025.
Declarations
Authors’ contributions: FO, EA, conceived the original idea. EA, NO, DA, AT, OO performed the experiments. FO supervised the work. FO, MO, and NO analyzed the data. FO, MO, draft the original manuscript. FO, MO, reviewed the manuscript. All authors have read and approved the final manuscript.
- International Diabetes Federation. IDF Diabetes Atlas 2021. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021. [accessed 2023 Feb 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK581934/
- World Health Organization (WHO). Diabetes. [accessed 2022 Sep 16]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- Shojima N, Yamauchi T. Progress in genetics of type 2 diabetes and diabetic complications. J Diabetes Investig. 2023;14(4):503-15. Available from: https://doi.org/10.1111/jdi.13970
- González P, Lozano P, Ros G, Solano F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int J Mol Sci. 2023;24(11):9352. Available from: https://doi.org/10.3390/ijms24119352
- Caturano A, D'Angelo M, Mormone A, Russo V, Mollica MP, Salvatore T, et al. Oxidative stress in type 2 diabetes: Impacts from pathogenesis to lifestyle modifications. Curr Issues Mol Biol. 2023;45(8):6651-66. Available from: https://doi.org/10.3390/cimb45080420
- Ko CY, Xu JH, Lo YM, Tu RS, Wu JS, Huang WC, et al. Alleviative effect of alpha-lipoic acid on cognitive impairment in high-fat diet and streptozotocin-induced type 2 diabetic rats. Front Aging Neurosci. 2021;13:774477. Available from: https://doi.org/10.3389/fnagi.2021.774477
- Galizzi G, Di Carlo M. Insulin and its key role for mitochondrial function/dysfunction and quality control: a shared link between dysmetabolism and neurodegeneration. Biology. 2022;11:943. Available from: https://doi.org/10.3390/biology11060943
- Adeyomoye OI, Adetunji JB, Olaniyan OT, Adetunji CO, Ogunmiluyi OE. Effects of Ficus exasperata on neurotransmission and expression of BDNF, tau, ACHE, and BACE in diabetic rats. Metabolism Open. 2024;24:100333. Available from: https://doi.org/10.1016/j.metop.2024.100333
- Pathak R, Sachan N, Chandra P. Mechanistic approach towards diabetic neuropathy screening techniques and future challenges: A review. Biomed Pharmacother. 2022;150:113025. Available from: https://doi.org/10.1016/j.biopha.2022.113025
- Petersen EA, Stauss TG, Scowcroft JA, Jaasma MJ, Brooks ES, Edgar DR, et al. Long-term efficacy of high-frequency (10 kHz) spinal cord stimulation for the treatment of painful diabetic neuropathy: 24-Month results of a randomized controlled trial. Diabetes Res Clin Pract. 2023;203:110865. Available from: https://doi.org/10.1016/j.diabres.2023.110865
- Ang L, Mizokami-Stout K, Eid SA, Elafros M, Callaghan B, Feldman EL, et al. The conundrum of diabetic neuropathies, present and future. J Diabetes Complicat. 2022;36(11):108334. Available from: https://doi.org/10.1016/j.jdiacomp.2022.108334
- Hurtado-Carneiro V, LeBaut-Ayuso Y, Velázquez E, Flores-Lamas C, Fernández-de la Rosa R, García-García L, et al. Effects of chronic treatment with metformin on brain glucose hypometabolism and central insulin actions in transgenic mice with tauopathy. Heliyon. 2024;10(15):e35752. Available from: https://doi.org/10.1016/j.heliyon.2024.e35752
- Singh AD, Chawda M, Kulkarni YA. Vasant Kusumakar Rasa ameliorates diabetic encephalopathy by reducing oxidative stress and neuroinflammation and improving neurotransmitter levels in experimental animals. Cureus. 2024;16(12):e75905. Available from: https://doi.org/10.7759/cureus.75905
- Arora T, Tremaroli V. Therapeutic potential of butyrate for treatment of type 2 diabetes. Front Endocrinol (Lausanne). 2021;12:761834. Available from: https://doi.org/10.3389/fendo.2021.761834
- Chen XF, Chen X, Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci (Lond). 2020;134:657-76. Available from: https://doi.org/10.1042/cs20200128
- Xiong RG, Zhou DD, Wu SX, Huang SY, Saimaiti A, Yang ZJ, et al. Health benefits and side effects of short-chain fatty acids. Foods. 2022;11(18):2863. Available from: https://doi.org/10.3390/foods11182863
- Zheng J, An Y, Du Y, Song Y, Zhao Q, Lu Y. Effects of short-chain fatty acids on blood glucose and lipid levels in mouse models of diabetes mellitus: A systematic review and network meta-analysis. Pharmacol Res. 2024;199:107041. Available from: https://doi.org/10.1016/j.ph.2023.107041
- Mandaliya DK, Patel S, Seshadri S. The combinatorial effect of acetate and propionate on high-fat diet-induced diabetic inflammation or metaflammation and T cell polarization. Inflammation. 2021;44(1):68-79. Available from: https://doi.org/10.1007/s10753-020-01309-7
- Wu Q, Dong J, Bai X, Jiang Y, Li J, Fan S, et al. Propionate ameliorates diabetes-induced neurological dysfunction through regulating the PI3K/Akt/eNOS signaling pathway. Eur J Pharmacol. 2022;925:174974. Available from: https://doi.org/10.1016/j.ejphar.2022.174974
- Pyo YH, Lee DB, Lee YW, Yoon SM, Lee AR. Hypoglycemic and hypolipogenic action of acetic acid and monascus-fermented grain vinegar: a comparative study. J Med Food. 2022;25(4):418-25. Available from: https://doi.org/10.1089/jmf.2021.K.0156
- Naif AR, Al-Abbasi AA, Moglad E, Afzal M, Al-Qahtani SD, Alzarea SI, et al. Protective activity of hirsutidin in high-fat intake and streptozotocin-induced diabetic rats: In silico and in vivo study. Heliyon. 2024;10(19):e38625. Available from: https://doi.org/10.1016/j.heliyon.2024.e38625
- Kumari S, Kamboj A, Wanjari M, Sharma AK. Nephroprotective effect of Vanillic acid in STZ-induced diabetic rats. J Diabetes Metab. 2021;20(1):571-582. Available from: https://doi.org/10.1007/s40200-021-00782-7
- Dayarathne LA, Ranaweera SS, Natraj P, Rajan P, Lee YJ, Han CH. The effects of naringenin and naringin on the glucose uptake and AMPK phosphorylation in high glucose-treated HepG2 cells. J Vet Sci. 2021;22(6):e92. Available from: https://doi.org/10.4142/jvs.2021.22.e92
- Røikjer J, Mørch CD, Ejskjaer N. Diabetic peripheral neuropathy: diagnosis and treatment. Curr Drug Saf. 2021;16(1):2–16. Available from: https://doi.org/10.2174/157488631566620073117311
- Ebokaiwe AP, Osawe S, Griffin S, Keck CM, Olusanya O, Ehiri RC. Loranthus micranthus nanoparticles abate streptozotocin-instigated testicular dysfunction in Wistar rats: Involvement of glucose metabolism enzymes, oxidative-inflammatory stress, steroidogenic enzymes/protein, and Nrf2 pathway. Andrologia. 2020;52(10):e13749. Available from: https://doi.org/10.1111/and.13749
- Yuliani T, Lobentanzer S, Klein J. Central cholinergic function and metabolic changes in streptozotocin-induced rat brain injury. J Neurochem. 2021;158(6):1307-1319. Available from: https://doi.org/10.1111/jnc.15155
- Dubey SK, Lakshmi KK, Krishna KV, Agrawal M, Singhvi G, Saha RN, et al. Insulin-mediated novel therapies for the treatment of Alzheimer's disease. Life Sci. 2020;249:117540. Available from: https://doi.org/10.1016/j.lfs.2020.117540
- Swamy BK, Shiprath K, Ratnam. Electrochemical detection of dopamine and tyrosine using metal oxide (MO, M=Cu and Ni) modified graphite electrode: a comparative study. Biointerface Res Appl Chem. 2020;10(5):6460-6473. Available from: http://dx.doi.org/10.33263/BRIAC105.64606473
- Graves SM, Xie Z, Stout KA, Zampese E, Burbulla LF, Shih JC, et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat Neurosci. 2020;23(1):15-20. Available from: https://doi.org/10.1038/s41593-019-0556-3
- Wang X, He G, Peng Y, Zhong W, Wang Y, Zhang B, et al. Sodium butyrate alleviates adipocyte inflammation by inhibiting the NLRP3 pathway. Sci Rep. 2015;3(5):12676. Available from: https://www.nature.com/articles/srep12676
- Schröder M, Pasic A, Hirche F, Rozanova S, Sgodzai M, Gisevius B, et al. Oral supplementation with propionate is reflected in the serum of healthy individuals. Ther Adv Neurol Disord. 2025;18. Available from: https://journals.sagepub.com/doi/10.1177/17562864241309755
- Hou YF, Shan C, Zhuang SY, Zhuang QQ, Ghosh A, Zhu KC, et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease. Microbiome. 2021;9(1):34. Available from: https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-020-00988-6
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. Available from: https://pubmed.ncbi.nlm.nih.gov/4337382/
- Matar S, Gomaa RA, El Wakil A, Essawy A. Human placental extract rescues hippocampal damage associated with cognitive impairment in diabetic male rats through anti-oxidative, anti-inflammatory, and neuromodulatory activities. Metab Brain Dis. 2025;40(6):225. Available from: https://link.springer.com/article/10.1007/s11011-025-01646-2
- Komleva Y, Chernykh A, Lopatina O, Gorina Y, Lokteva I, Salmina A. Inflamm-aging and brain insulin resistance: New insights and role of life-style strategies on cognitive and social determinants in aging and neurodegeneration. Front Neurosci. 2021;14:618395. Available from: https://www.frontiersin.org/articles/10.3389/fnins.2020.618395/full
- Meng L, Li XY, Shen L, Ji HF. Type 2 diabetes mellitus drugs for Alzheimer's disease: Current evidence and therapeutic opportunities. Trends Mol Med. 2020 Jun;26(6):597–614. Available from: https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(20)30032-3
- Swain SK, Chandra Dash U, Sahoo AK. Hydrolea zeylanica improves cognitive impairment in high-fat diet fed-streptozotocin-induced diabetic encephalopathy in rats via regulating oxidative stress, neuroinflammation, and neurotransmission in brain. Heliyon. 2022;8(11):e11301. Available from: https://www.cell.com/heliyon/fulltext/S2405-8440(22)02608-6
- Srisuksai K, Parunyakul K, Phaonakrop N, Roytakul S, Fungfuang W. The effect of cordycepin on brain oxidative stress and protein expression in streptozotocin-induced diabetic mice. J Vet Med Sci. 2021;83(9):1425–1434. Available from: https://www.jstage.jst.go.jp/article/jvms/83/9/83_21-0268/_article
- Lerner TN, Holloway AL, Seiler JL. Dopamine, updated: reward prediction error and beyond. Curr Opin Neurobiol. 2021;67:123–30. Available from: https://doi.org/10.1016/j.conb.2020.10.012
- Al-Hafidh SHA, Abdulwahid AA. Neurotoxic effects of type II diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. Open Vet J. 2024;14(10):2651-2661. Available from: https://doi.org/10.5455/OVJ.2024.v14.i10.15
- Jiang Y, Zou D, Li Y, Gu S, Dong J, Ma X, et al. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals. 2022;15(10):1203. Available from: https://doi.org/10.3390/ph15101203
- Okesola MA, Ajiboye BO, Oyinloye BE, Osukoya OA, Owero-Ozeze OS, Ekakitie LI, et al. Effect of Solanum macrocarpon Linn leaf aqueous extract on the brain of an alloxan-induced rat model of diabetes. J Int Med Res. 2020;48(6):300060520922649. Available from: https://doi.org/10.1177/0300060520922649
- Ajiboye BO, Ekundayo BE, Salami AW, Osukoya AO, Komolafe K, Gaur S, et al. Neuroprotective effect of Lannea egregia alkaloid-rich leaf extracts in streptozotocin-induced diabetic rats. Toxicol Rep. 2024;13:101742. Available from: https://doi.org/10.1016/j.toxrep.2024.101742
- Arshad MS, Imran M, Ahmed A, Sohaib M, Ullah A, Nisa MU, et al. Tamarind: a diet-based strategy against lifestyle maladies. Food Sci Nutr. 2019;7(11):3378-3390. Available from: https://doi.org/10.1002/fsn3.1218
- Makrecka-Kuka M, Liepinsh E, Murray AJ, Lemieux H, Dambrova M, Tepp K, et al. Altered mitochondrial metabolism in the insulin-resistant heart. Acta Physiol. 2020;228(3):e13430. Available from: https://doi.org/10.1111/apha.13430
- Kim KY, Shin KY, Chang KA. Potential biomarkers for post-stroke cognitive impairment: a systematic review and meta-analysis. Int J Mol Sci. 2022;23(2):602. Available from: https://doi.org/10.3390/ijms23020602
- Deng X, Saffari SE, Ng SYE, Chia N, Tan JY, Choi X, et al. Blood lipid biomarkers in early Parkinson's disease and Parkinson's disease with mild cognitive impairment. J Parkinsons Dis. 2022;12(6):1937-1943. Available from: https://doi.org/10.3233/JPD-213135
- Saeed A, Lopez O, Cohen A, Reis SE. Cardiovascular disease and Alzheimer's disease: the heart-brain axis. Am Heart J. 2023;12(21):e030780. Available from: https://doi.org/10.1161/JAHA.123.030780
- Nagayach A, Bhaskar R, Ghosh S, Singh KK, Han SS, Sinha JK. Advancing the understanding of diabetic encephalopathy through unravelling pathogenesis and exploring future treatment perspectives. Ageing Res Rev. 2024;100:102450. Available from: https://doi.org/10.1016/j.arr.2024.102450
- Rahman MM, Dhar PS, Anika SF, Anika F, Ahmed L, et al. Exploring the plant-derived bioactive substances as antidiabetic agents: an extensive review. Biomed Pharmacother. 2022;152:113217. Available from: https://doi.org/10.1016/j.biopha.2022.113217
- Tian Z, Ji X, Liu J. Neuroinflammation in vascular cognitive impairment and dementia: current evidence, advances, and prospects. Int J Mol Sci. 2022;23(11):6224. Available from: https://doi.org/10.3390/ijms23116224
- Huang Q, Liao C, Ge F, Ao J, Liu T. Acetylcholine bidirectionally regulates learning and memory. J Neurorestoratology. 2022;10(2):100002. Available from: https://doi.org/10.1016/j.jnrt.2022.100002
- Esmaeili MH, Enayati M, Khabbaz Abkenar F, Ebrahimian F, Salari AA. Glibenclamide mitigates cognitive impairment and hippocampal neuroinflammation in rats with type 2 diabetes and sporadic Alzheimer-like disease. Behav Brain Res. 2020;379:112359. Available from: https://doi.org/10.1016/j.bbr.2019.112359
- Gelen V, Şengül E, Yıldırım S, Senturk E, Tekin S, Kükürt A. The protective effects of hesperidin and curcumin on 5-fluorouracil-induced nephrotoxicity in mice. Environ Sci Pollut Res Int. 2021;28(34):47046-47055. Available from: https://doi.org/10.1007/s11356-021-13969-5
- Gezer A, Karadağ Sarı E, Gelen V, et al. Exploring the neuroprotective effects of black garlic ethanol extract on acrylamide-induced brain damage through apoptotic and neurodegenerative pathways. Ankara Univ Vet Fak Der. 2024;71(4):395-406. Available from: https://doi.org/10.33988/auvfd.1384531
- Lin ZX, Wang CJ, Tu HW, Tsai MT, Yu MH, Huang HP, et al. The neuroprotective effects of primary functional components mulberry leaf extract in diabetes-induced oxidative stress and inflammation. J Agric Food Chem. 2025;73(6):3680-3691. Available from: https://doi.org/10.1021/acs.jafc.4c09422
- Nasiry D, Khalatbary AR, Ahmadvand H, Talebpour Amiri F, Akbari E. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Complement Altern Med. 2017;17(1):476. Available from: https://doi.org/10.1186/s12906-017-1983-x